Questions by:
Pam Brown, GP, Swansea
Jane Diggle, Specialist Diabetes Nurse Practitioner, West Yorkshire
Q: Should we perform a full lipid profile as an initial baseline and/or for ongoing monitoring? Should this be a fasted profile?
From the point of view of accuracy, it is advisable to perform a full fasted lipid profile at least for an initial baseline, and ideally for subsequent monitoring testing as well. However, NICE guidance advises using non-fasted samples as they are generally more convenient, stating, “a fasting sample is not mandated” (NICE, 2023a). There is no clear consensus around use of fasted profiles for monitoring.
A full lipid profile involves taking a blood sample to measure total cholesterol, HDL cholesterol and triglyceride levels, and then calculating non-HDL cholesterol and LDL cholesterol levels. LDL is usually calculated using the Friedewald formula (Friedewald et al, 1972). Lipid profiles may also include calculated values for total cholesterol:HDL ratio and for non-HDL cholesterol. Depending on local arrangements, biochemistry laboratories may provide a full profile for all requests or they may omit triglycerides from non-fasted samples.
Fasted samples refer to those which are taken following overnight dietary restriction for 12–14 hours, allowing only consumption of water and medication. This is preferable because triglycerides can be elevated postprandially for several hours (Campos et al, 2005), and lipid reference range values are derived from fasted individuals (NCEP Expert Panel, 2002). Total cholesterol and HDL cholesterol concentrations are less affected by fasting and can be used in the non-fasted state in most cases. Conversely, calculation of LDL cholesterol is critically dependent on a fasted triglyceride value; thus, if non-fasted triglycerides are measured then the calculated LDL is likely to be significantly underestimated, which could adversely affect management. As such, it is worth noting that LDL cholesterol results may not be reported in participants with triglyceride levels more than 4.5 mmol/L or 9 mmol/L depending on the formula used by local laboratories.
Non-fasted triglyceride values in themselves can have some value for cardiovascular risk prediction, as they can provide an insight into an individual’s ability to clear triglyceride-rich lipoprotein particles from the circulation (Nordestgaard et al, 2007). However, no reliable reference ranges exist for this indication, which makes interpretation of results challenging.
Q: What other blood tests should be done when assessing for dyslipidaemia?
Alongside a full lipid profile, the following should be measured as part of the initial baseline assessment (NICE, 2023b):
- Creatinine kinase – if the patient has persistent generalised unexplained muscle pain. This should not be measured routinely, especially if the person is asymptomatic.
- Liver function tests: alanine aminotransferase or aspartate aminotransferase and albumin.
- Renal function – including eGFR.
- HbA1c – to check for diabetes diagnosis.
- Thyroid stimulating hormone – in people with symptoms of hyper- or hypothyroidism.
Renal, thyroid and liver profiles and HbA1c are required to exclude secondary causes of dyslipidaemia and comorbidities.
Q: What are the current recommended lipid targets for primary and secondary prevention of cardiovascular disease?
The NICE (2023a) NG238 guideline provides lipid lowering targets for primary and secondary prevention of cardiovascuar disease (CVD). For primary prevention, aim for a >40% reduction in non-HDL cholesterol.
For secondary prevention of CVD, aim for an LDL of 2.0 mmol/L or less, or a non-HDL of 2.6 mmol/L or less.
Q: Should we request triglycerides before initiating incretin-based drugs in those with high HbA1c?
People with type 2 diabetes should have regular monitoring of their lipid parameters, including triglycerides, as part of their routine care. Extreme hypertriglyceridaemia is a risk factor for acute pancreatitis, but the risk tends to only increase significantly once concentrations exceed 10–20 mmol/L (Kota et al, 2012). GLP-1 receptor agonists are also rarely associated with acute pancreatitis; however, the exact nature of this relationship is unclear. Patients should be informed of the characteristic symptoms of acute pancreatitis – abdominal pain, nausea, vomiting and fever. If pancreatitis is suspected, the GLP-1 agent should be discontinued and if confirmed should not be restarted.
It would be prudent to exercise caution in those with known significant hypertriglyceridaemia, a family history of pancreatitis or risk factors for pancreatitis. Risk factors include high alcohol intake, gallstones and hypertriglyceridaemia (Davies, 2022; Milne, 2023). This should occur regardless of HbA1c status.
Q: Triglycerides are elevated in association with high HbA1c and poor glycaemic control. Is this a direct relationship or is there an HbA1c threshold above which triglycerides rise?
The relationship between HbA1c and triglyceride concentration is complex, and there are many other factors involved in determining an individual’s triglyceride concentration. These include genetic factors, as some individuals are better able to clear triglycerides than others, as well as secondary factors such as alcohol intake, BMI and body composition, and medications (Box 1). It is clear that, as HbA1c increases, so too does the risk of severe hypertriglyceridaemia; however, the HbA1c level at which this happens is highly variable.
Q: What constitutes severe hyperlipidaemia and how should this be investigated?
Adults with a total cholesterol >7.5 mmol/L, an LDL cholesterol >4.9 mmol/L and/or a non-HDL cholesterol >5.9 mmol/L can be considered to have severe hyperlipidaemia. In people with a personal and/or family history of confirmed coronary heart disease (under the age of 60 years), and with no secondary causes, familial hypercholesterolaemia (FH) should be suspected.
People who have possible or definite FH are at high risk of CVD, and as such use of conventional risk calculation tools, such as QRISK, is not validated or therefore advised. A fasting blood sample should be taken to repeat the lipid profile and measure LDL.
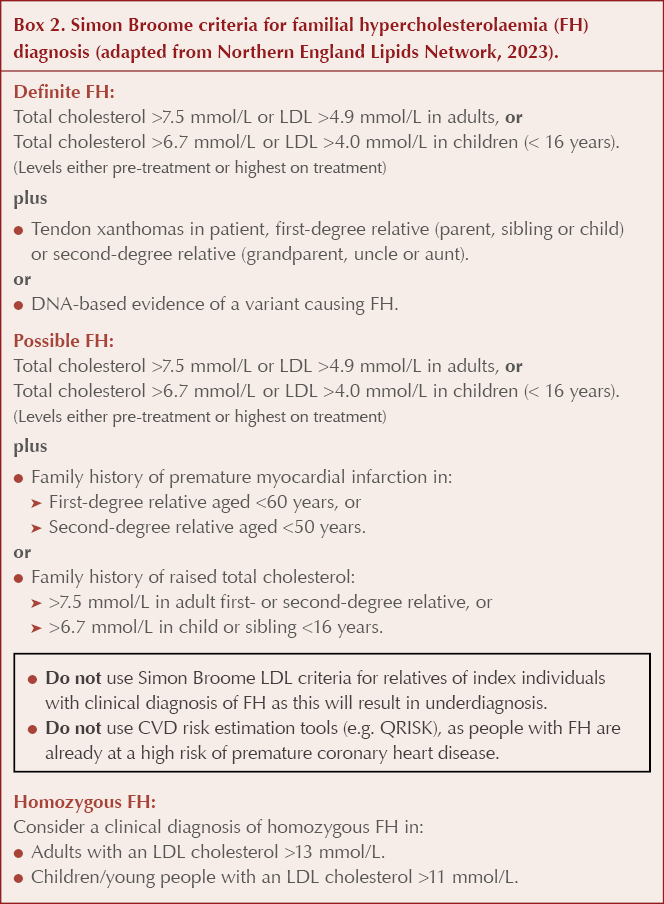
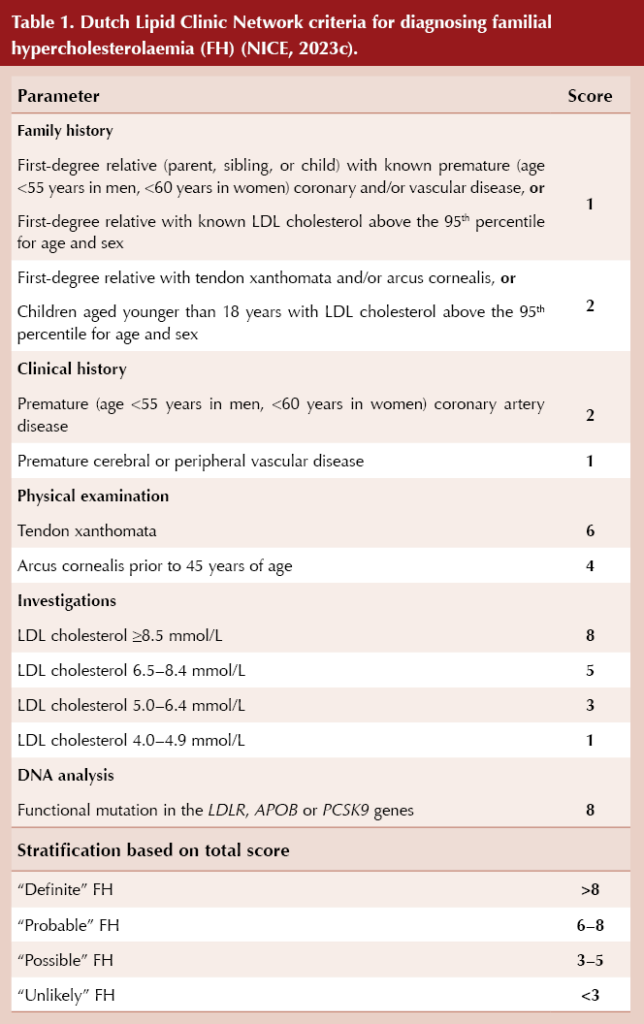
The Simon Broome criteria (Box 2) or the Dutch Lipid Clinic Network criteria (Table 1) should be used to aid diagnosis of FH (NICE, 2019). The clinical findings, a full lipid profile and family history should be used collaboratively to judge the likelihood of a familial lipid disorder, rather than using a strict lipid cut-off value alone.
In addition to a clinical diagnosis of FH, the following scenarios warrant referral of the individual to a specialist lipid clinic for further assessment, irrespective of family history:
- Total cholesterol >9 mmol/L.
- LDL cholesterol >6.5 mmol/L.
- Non-HDL cholesterol >7.5 mmol/L.
- Fasting triglycerides >10 mmol/L.
Q: What are the common secondary causes of severe hyperlipidaemia?
Chronic renal failure in itself can lead to impaired clearance of triglycerides and raised serum triglyceride concentrations. Nephrotic syndrome is associated with more profound lipid abnormalities, the magnitude of which is associated with the extent of proteinuria (Vaziri, 2016). There are increases seen in total cholesterol, triglycerides and LDL cholesterol, as well as increased postprandial hyperlipidaemia. HDL levels are less affected, and can even be reduced (Joven et al, 1990). These abnormalities can result in accelerated atherosclerosis and progression of cardiovascular disease.
Type 2 diabetes and obesity are associated with hypertriglyceridaemia, which can be extreme but generally has less impact on total cholesterol. LDL cholesterol values should be unchanged, but calculated LDL will not be possible in the context of significantly raised triglycerides.
Excessive alcohol intake is also commonly associated with an increase in triglycerides, and this can be extreme, particularly when it occurs alongside an additional secondary factor such as type 2 diabetes or obesity.
Hypothyroidism can lead to marked hyperlipidaemia, with increases predominantly seen in LDL cholesterol. However, it is important to note that this will not affect the majority of patients with hypothyroidism, and only tends to be seen in extreme cases.
Cholestasis can result in severe hyperlipidaemia due to accumulation of lipoprotein X (Brandt et al, 2015). In a conventional lipid profile, this will manifest as a markedly elevated calculated LDL cholesterol result, but lipoprotein X is in fact a structurally separate lipoprotein (Seidel et al, 1969).
Several drug classes are associated with hyperlipidaemia, including corticosteroids, beta-blockers, thiazide diuretics, isotretinoin and the oral contraceptive pill.
Q: What effect does a ketogenic diet have on the lipid profile and does this significantly change cardiovascular risk?
A ketogenic diet severely restricts carbohydrates, with no restrictions on fats. The principle behind this diet is to use fat as a fuel source in preference to carbohydrates.
The US National Lipid Association Nutrition and Lifestyle Task Force has reviewed the evidence on ketogenic diets and published a scientific statement (Kirkpatrick et al, 2019). The authors concluded that ketogenic diets show variable effects on LDL cholesterol levels, with some studies showing an increase driven by high saturated fat intake, and by individual genetic factors. As a result of this, ketogenic diets should be contraindicated in people with hypercholesterolaemia, particularly FH, and in people with severe hypertriglyceridaemia due to genetic or acquired causes of lipoprotein lipase dysfunction.
Ketogenic diets have shown inconsistent effects on other cardiometabolic risk factors such as HbA1c and blood pressure, whilst a Mediterranean diet showed greater improvements in triglycerides and HDL cholesterol compared with low-carbohydrate diets in individuals with type 2 diabetes. Observational studies suggest that both low and high carbohydrate intakes are associated with increased risk of cardiovascular mortality compared with moderate intake.
Overall, the effect of ketogenic diets on long-term cardiovascular risk remains unknown and, therefore, they are not recommended in national guidance, as they severely restrict or eliminate foods that are associated with cardioprotective benefits and encourage a high intake of those known to increase cardiovascular risk (Heart UK, 2024).
Q: What dietary options are recommended for people at high cardiovascular risk?
NICE guidance on lipid modification has been updated and now gives greater detail regarding dietary advice for those with or at high risk of CVD. NICE (2023a) advises a diet in which total fat intake is ≤30% of total energy intake, saturated fats are ≤7% of total energy intake and, where possible, saturated fats are replaced by mono-unsaturated and polyunsaturated fats. Additionally, people are advised to reduce their saturated fat intake and to increase their mono-unsaturated fat intake by using olive oil and rapeseed oil, or spreads based on these, in food preparations.
HEART UK has a range of resources around dietary intake linked to cholesterol, including advice on the cholesterol content of foods, diet plans and more. These can be a good resource to signpost to.
Acknowledgement
This Q&A was originally authored by Patrick Wainwright in 2022. Claire Davies has revised the answers in response to updated NICE recommendations published in December 2023.




Small but significant 12% increased risk of developing chronic cough compared to treatment with other second-line agents for type 2 diabetes.
8 Dec 2025