Update history: Updated on 22 July 2025 to reflect new NICE TAs on dapagliflozin/empagliflozin for CKD/HFpEF in algorithm.
What are SGLT2 inhibitors?
Sodium–glucose cotransporter 2 (SGLT2) inhibitors, also known as “Flozins”, are a class of medication primarily used to manage type 2 diabetes. These drugs help lower blood glucose by targeting the kidneys and are also recognised for their protective effects on the heart and kidneys, often used in managing heart failure and chronic kidney disease.1,2
Mechanisms of action1
SGLT2 inhibitors work by reversibly inhibiting the sodium–glucose cotransporter 2 protein in the renal proximal convoluted tubule. This inhibition reduces reabsorption of glucose back into the blood, causing excess glucose to be excreted in the urine. This mechanism helps to lower blood glucose levels and, independently, provides cardiovascular and renal protective benefits.
Although the mechanisms behind the improved outcomes seen are not fully understood, they are hypothesised to include natriuresis and plasma volume reductions, which lower blood pressure and cardiac workload, and improved vascular function. Renal benefits involve reduced hyperfiltration and albuminuria, which protect kidney function. Additionally, the drugs shift metabolism to ketone bodies for heart and kidney energy, reduce inflammation and oxidative stress, and lower serum uric acid levels. These effects, alongside suppressed advanced glycation end-products, support potential broad organ protection. Studies exploring applications in non-diabetic kidney disease and heart failure with preserved ejection fraction are ongoing.
Licensed indications
Type 2 diabetes
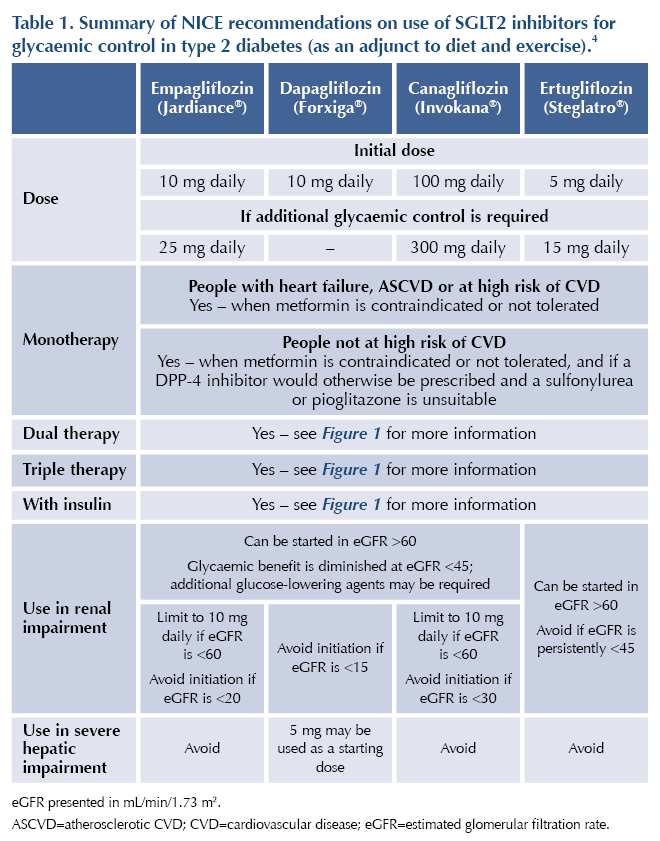
All four SGLT2 inhibitors available in the UK are licensed for the treatment of adults with insufficiently controlled type 2 diabetes, as an adjunct to diet and exercise. This class of drugs can be prescribed as monotherapy where metformin is not appropriate or tolerated, as well as in combination with other glucose-lowering agents.
Heart failure
Dapagliflozin and empagliflozin are indicated for the treatment of symptomatic chronic heart failure regardless of ejection fraction in adults with or without type 2 diabetes. See NICE NG106 for further information.2
Chronic kidney disease
Dapagliflozin and empagliflozin are licensed for the treatment of chronic kidney disease in adults without type 2 diabetes. See NICE NG203 for further information.3
Canagliflozin is indicated for the treatment of kidney disease in adults but only in the presence of type 2 diabetes.
Positioning in guidelines
NICE NG28 advocates the use of SGLT2 inhibitors as second-line pharmacotherapy in addition to metformin for people who have established cardiovascular disease (CVD) or have a high risk of CVD (QRISK ≥10%).4 See Table 1 and Figure 15 for more information.

Principal effects
Glycaemic control
SGLT2 inhibitors reduce glucose levels by preventing the reabsorption of glucose through the urinary tract. They can be used alone or with other agents for a synergistic glucose-lowering effect at licensed doses.
Additional benefits6,7
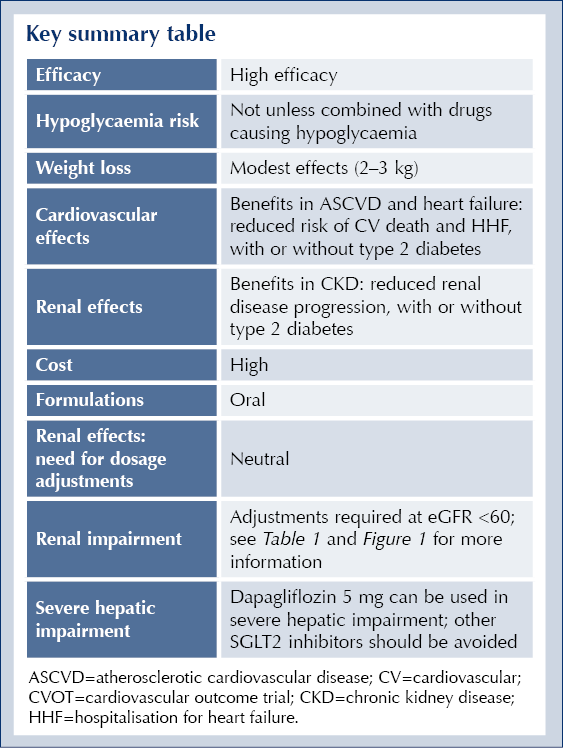
Reduction in body weight: Provide a modest but consistent weight reduction, typically ranging between 2–3 kg in clinical trials. This weight loss effect is generally attributed to increased urinary glucose excretion and slight reductions in body fluid volume.
Reduction in kidney disease progression: Reduce the risk of kidney disease progression by 37% in patients with chronic kidney disease, whether they have diabetes or not.
Reduction in cardiovascular death or hospitalisation for heart failure: Reduce the combined risk of cardiovascular death and hospitalisation for heart failure by 23% (relative risk [RR] 0.77; 95% CI 0.74–0.81). These benefits were consistent in people with and without diabetes, with relative risks of 0.77 in those with diabetes and 0.79 in those without.
Reduction in cardiovascular death: Also lower the risk of cardiovascular death alone by 14% (RR 0.86; 95% CI 0.81–0.92), showing similar effects between people with and without diabetes.
Contraindications
● Type 1 diabetes due to an increased risk of diabetic ketoacidosis.
● Severe renal impairment: avoid in people with an eGFR below the specific cut-offs stated in Table 1.
● Hypersensitivity to any SGLT2 inhibitor or to any of their excipients.
● Current or recent diabetic ketoacidosis, due to the risk of worsening this condition.
● Pregnancy or breastfeeding: limited information.
Cautions
● Elderly population.
● Hypotension.
● Hypovolaemia or at risk of hypovolaemia.
● Complicated urinary tract infections (empagliflozin).
● Raised haematocrit (canagliflozin).
● People adhering to a ketogenic/low-calorie/low-carbohydrate diet.
● Glycaemic effect is reduced below eGFR 60 mL/min/1.73 m2: additional glucose-lowering agents may be required.
Adverse effects
Common: Increased risk of urinary infections, dizziness, urinary disorders, thirst, hypoglycaemia (when combined with insulin or sulfonylureas), skin reactions, genital infections.
Uncommon: Hypotension, hypovolaemia, angioedema.
Rare: Diabetic ketoacidosis, fracture risk, lower limb amputation, Fournier’s gangrene.
Drug interactions
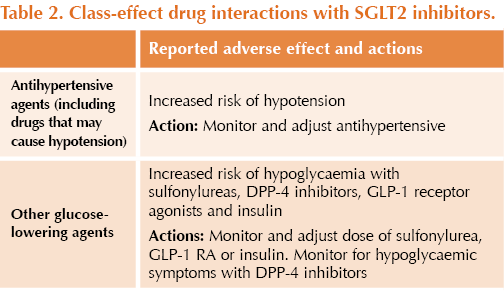
● There is an increased risk of hypotension when SGLT2 inhibitors are used with drugs that may cause hypotension.
● There is an increased risk of hypoglycaemia when SGLT2 inhibitors are used with other glucose-lowering agents (especially sulfonylureas and insulin).
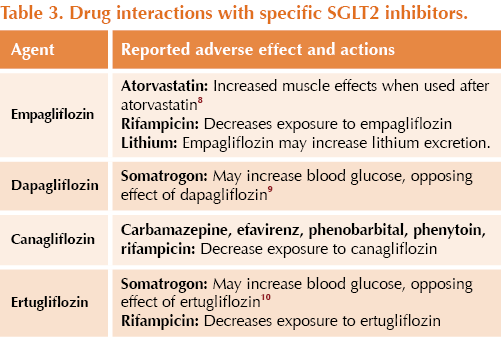
● Other drug specific interactions (not an exhaustive list) are stated in Table 2 and Table 3.
Monitoring
Renal function (eGFR)
● Monitor eGFR before initiating treatment and then at least annually thereafter, especially in people with renal impairment or those at risk of kidney dysfunction.
● An initial, reversible eGFR reduction of 5–8 mL/min/1.73 m² may occur within the first few weeks due to decreased hyperfiltration. This is normal.
Glycaemic control (HbA1c)
● Check HbA1c levels 3 months after starting treatment to evaluate efficacy, and then annually.
Ketones
● Monitor ketone levels during treatment interruptions, such as for surgery or acute serious medical conditions, to reduce the risk of euglycaemic diabetic ketoacidosis, even if blood glucose is near normal.
Signs and symptoms of diabetic ketoacidosis
● Educate patients to monitor for DKA symptoms, including nausea, vomiting, abdominal pain, excessive fatigue and difficulty breathing, and to seek urgent medical care if these symptoms appear.
Blood pressure
● Regularly assess blood pressure, as SGLT2 inhibitors can lower this; adjustments in antihypertensive medication may be required. Measure at baseline and then at least annually.
Hydration and blood volume status
● Monitor hydration status, especially in elderly patients or those with renal impairment, as SGLT2 inhibitors increase urinary excretion and may lead to dehydration or hypotension.
Body weight
● Track weight periodically, as weight loss is a common effect and may indicate improved metabolic control.
Tolerance
● Review side effects, adherence and sick day rule understanding at each review.
Prescribing tips
Minimising risk of hypoglycaemia
● Review glucose-lowering agents and consider dose reductions to reduce the likelihood of hypoglycaemia with concomitant use, especially if HbA1c is already at target.
Minimising risk of diabetic ketoacidosis
● Review DKA risk factors and address modifiable risk factors such as low-calorie or ketogenic diets. Note that DKA can occur with normal glucose levels with SGLT2 inhibitors. The risk of this is higher during acute illness.
Minimising risk of hypotension
● Review diuretic and antihypertensive therapies periodically if hypertension improves, and adjust doses accordingly.
Sick day rules
● Counsel on sick day rules and side effects upon initiation, and review patients’ understanding of how to identify features of acute illness periodically.
Additional safety information
● MHRA (April 2016): Updated advice on the risk of diabetic ketoacidosis
● MHRA (March 2017): Updated advice on increased risk of lower-limb amputations (mainly toes)
● MHRA (February 2019): Reports of Fournier’s gangrene (necrotising fasciitis of the genitalia or perineum)
● MHRA (March 2020): Monitor ketones in blood during treatment interruptions for surgical procedures or acute serious medical illness
Key summary table
See also
● Need to know guide: SGLT2 inhibitors: Indications, doses and licences in adults
● How to: How to use SGLT2 inhibitors safely and effectively





3-point MACE occurred in 12.2% vs 13.1% of tirzepatide and dulaglutide recipients, respectively, confirming non-inferiority.
19 Jan 2026