What is pioglitazone?
Pioglitazone is a thiazolidinedione, used as an insulin sensitiser in the management of type 2 diabetes.
Usage has declined somewhat over recent years, partly due to the emergence of newer classes of diabetes medications such as SGLT2 inhibitors and incretin-based therapies.1 Additionally, concerns about cardiovascular safety following the withdrawal of another thiazolidinedione, rosiglitazone, may have contributed to a decrease in pioglitazone use.2
However, pioglitazone remains a useful option for managing type 2 diabetes, particularly in people with metabolic-associated steatotic liver disease and/or insulin resistance.1
Available brands
Available generically as 15 mg, 30 mg or 45 mg tablets, taken once daily.
Available in combination with metformin as Competact tablets, containing pioglitazone 15 mg and metformin 850 mg, taken twice daily to achieve the recommended dose.
Mechanisms of action3
Pioglitazone is an agonist at peroxisome proliferator-activated receptors (PPARs), especially PPAR-gamma, which influence transcription of genes mediating carbohydrate and lipid metabolism. This results in:
● Increased insulin sensitivity in muscle, adipose tissue and the liver, resulting in enhanced glucose uptake and utilisation.
● Increased pancreatic sensitivity to glucose, resulting in optimised insulin secretion in response to high glucose levels.
● Suppressed liver gluconeogenesis.
● Reduced hepatic triglycerides.
● Reduced visceral fat mass and activity.
These effects result in an improvement in insulin sensitivity and lipid profile.
Licensed indications
● Monotherapy when metformin is not tolerated or is contraindicated.
● Dual therapy with metformin.
● Dual therapy with a sulfonylurea when metformin is not tolerated or is contraindicated.
● Triple therapy with metformin and a sulfonylurea.
● Can also be used alongside insulin when metformin is not tolerated or is contraindicated.
Positioning in guidelines
The NICE NG28 guideline4 places pioglitazone first-line when metformin and/or SGLT2 inhibitors are not tolerated or contraindicated. It can be used as a component of dual or triple oral therapy if mono or dual therapy has not achieved the individually agreed HbA1c target for a patient.
Although pioglitazone is not prominent in the ADA/EASD consensus report5, it is highlighted as a useful option for patients with metabolic dysfunction-associated steatotic liver disease (MASLD, previously known as NAFLD) and metabolic dysfunction-associated steatohepatitis (MASH, previously known as NASH), slowing progression to hepatic fibrosis.
Glycaemic effects
Pioglitazone improves insulin sensitivity in various tissues, including muscle, adipose tissue and the liver, leading to better glucose uptake and utilisation. This reduces both fasting and postprandial blood glucose levels.1 It is particularly effective in individuals with a high degree of insulin resistance, such as those with a high waist circumference or fatty liver.6 It has a moderately high efficacy, with HbA1c reductions of up to 14 mmol/mol observed.7
Because pioglitazone’s mode of action involves changes in gene transcription, glycaemic reduction is slower than with other agents and can require up to 3 months of treatment to take maximal effect; it is therefore not suitable as a rescue therapy. HbA1c should be measured 6 months after initiation and pioglitazone stopped if the reduction is less than 6 mmol/mol.8
Pioglitazone has a high durability of glycaemic influence, likely by preserving beta-cell function.1
Other benefits and cardiovascular safety
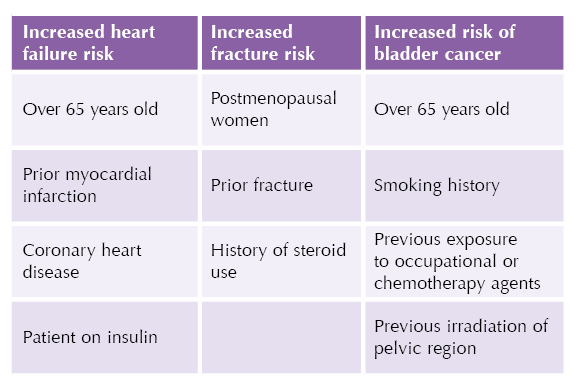
The PROactive trial showed that pioglitazone reduced the risk of ischaemic events, including non-fatal myocardial infarction and stroke, in people with pre-existing cardiovascular disease. However, this was offset by an increased risk of oedema and heart failure.9
However, a more recent review and analysis suggests that combination of pioglitazone with a GLP-1 receptor agonist or SGLT2 inhibitor is associated with weight loss and a reduced risk of heart failure.10
The IRIS trial similarly showed reduced incidence of further cardiovascular events following a stroke or myocardial infarction, although it should be noted that this trial included people with insulin resistance but not type 2 diabetes.11
Pioglitazone has been shown to slow progression of MASLD and MASH.12,13
Adverse effects
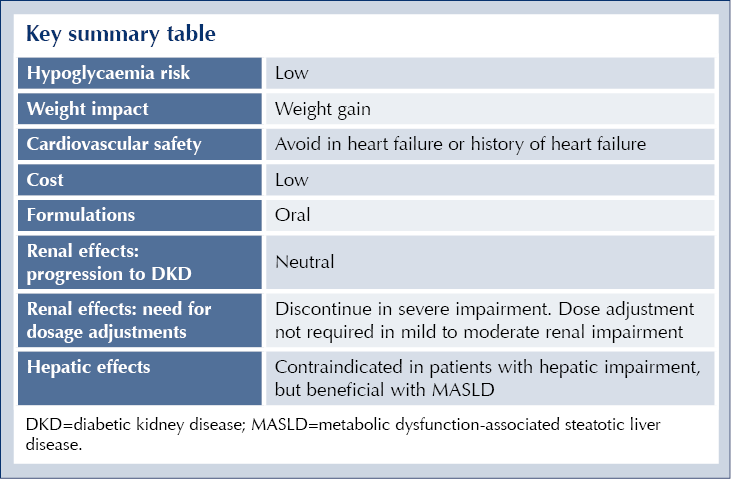
● Weight gain (mean increase of 3.6 kg in the PROactive study).9
● Numbness.
● Increased risk of infection.
● Visual impairment.
● Increased fracture risk.
● Increased bladder cancer risk.
● Hypoglycaemia if co-administered with insulin, sulfonylureas or meglitinides.
Contraindications
● Hypersensitivity to pioglitazone or other tablet ingredients.
● Heart failure or history of heart failure.
● Hepatic impairment.
● Diabetic ketoacidosis.
● Bladder cancer, history of bladder cancer or uninvestigated haematuria.
Cautions
● Pioglitazone can cause fluid retention, which can exacerbate or precipitate heart failure.
- Monitor for heart failure, oedema or rapid weight gain, especially in people with a history of myocardial infarction or coronary heart disease, and in the elderly.
- Pioglitazone should be started on the lowest 15 mg dose for these patients, and the dose increased cautiously.
- Fluid retention and heart failure risk increases further if pioglitazone is given alongside insulin.
● Pioglitazone has been associated with an increased risk of bladder cancer, although absolute risk remains low.
● Pioglitazone is associated with an increased risk of bone fracture; therefore, use with caution in post-menopausal women and people with a history of fracture.
● Be alert to the possibility of macular oedema if patients report disturbances in visual acuity; an appropriate ophthalmological referral should be considered.
Drug interactions
Pioglitazone is metabolised primarily by CYP450, CYP3A4 and CYP2C8.14 Inhibitors of these enzymes, such as gemfibrozil, clopidogrel, ketoconazole and trimethoprim may increase the risk of pioglitazone dose-related side effects.
Rifampicin is an inducer of both CYP3A4 and CYP2C8, so co-administration may result in reduced plasma levels and reduced efficacy of pioglitazone.
Addition of pioglitazone to a treatment regimen that includes insulin, meglitinides or sulfonylureas is likely to increase risk of hypoglycaemia.
Initiating pioglitazone
Pioglitazone can be started at 15 mg or 30 mg daily.
Prior to initiation, assess risk of heart failure, bone fracture and bladder cancer (see Table). In patients at higher risk, start with the lower 15 mg dose and monitor carefully, or consider alternative agents.
Check LFTs before initiation. Do not initiate if ALT is more than 2.5-times the upper limit of normal, or if there are any other signs of liver disease.
Dipstick urinalysis should be performed before initiation to rule out haematuria. If present, do not start pioglitazone until investigated.
If adding pioglitazone to insulin, meglitinide or sulfonylurea treatment, consider whether a dose reduction of those agents is needed to reduce risk of hypoglycaemia.
People initiated on pioglitazone must be warned to promptly report any symptoms of heart failure (rapid weight gain, shortness of breath) or bladder cancer (haematuria or urinary symptoms).
Monitoring
● Check LFTs within 3 months of initiation.
- Stop if ALT is more than 3-times the upper limit of normal range.
● Monitor for rapid weight gain, oedema, dyspnoea or fatigue, which may indicate heart failure.
- Stop pioglitazone immediately if there is any reason to suspect deterioration in heart function.
● Periodically review for symptoms of bladder cancer.
● Review response to treatment after 3–6 months, keeping in mind that pioglitazone has a slower onset of action than other agents.
- Stop if no substantial glycaemic improvement after 6 months of treatment.
Prescribing tips
● Pioglitazone is likely to be most effective in people with a high degree of insulin resistance, such as those with a large waist circumference.6
● Coadministration with an SGLT2 inhibitor or a GLP-1 receptor agonist may reduce weight gain and heart failure risk.10
Resources
● NICE Clinical Knowledge Summary: Pioglitazone
● Patient.info: Pioglitazone tablets for diabetes
Key summary




Small but significant 12% increased risk of developing chronic cough compared to treatment with other second-line agents for type 2 diabetes.
8 Dec 2025