What is a DPP-4 inhibitor?
Dipeptidyl peptidase-4 (DPP-4) inhibitors, also known as gliptins, are used in the management of type 2 diabetes. They inhibit the enzyme DPP-4, thus impairing the rapid degradation of two key incretin hormones: glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).1 DPP-4 plays a role in glucose homoeostasis by increasing nutrient-stimulated insulin synthesis and by reducing glucagon secretion. DPP-4 inhibitors double the circulating concentrations of both GLP-1 and GIP.2
The effects of the DPP-4 inhibitors are glucose-dependent and so there is a low risk of hypoglycaemia. The increased concentration of GLP-1 produced leads to reductions in prandial glucose excursions.
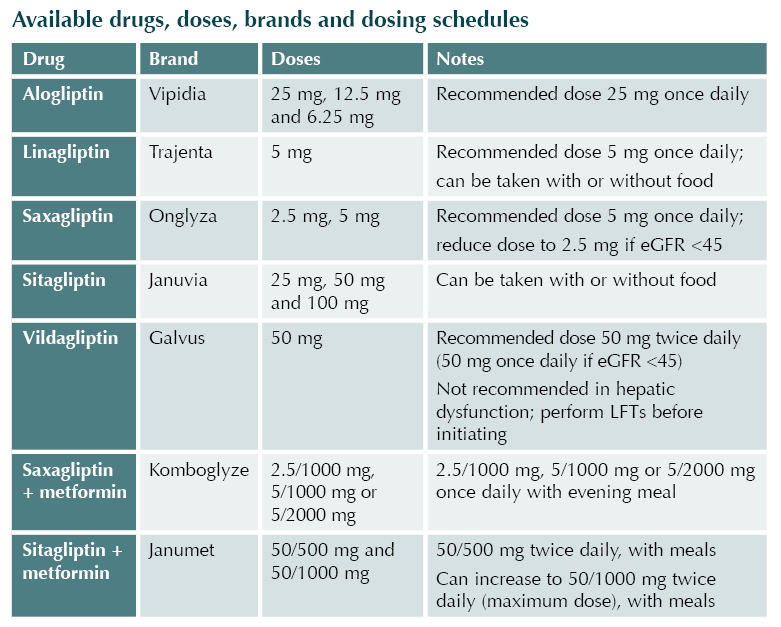
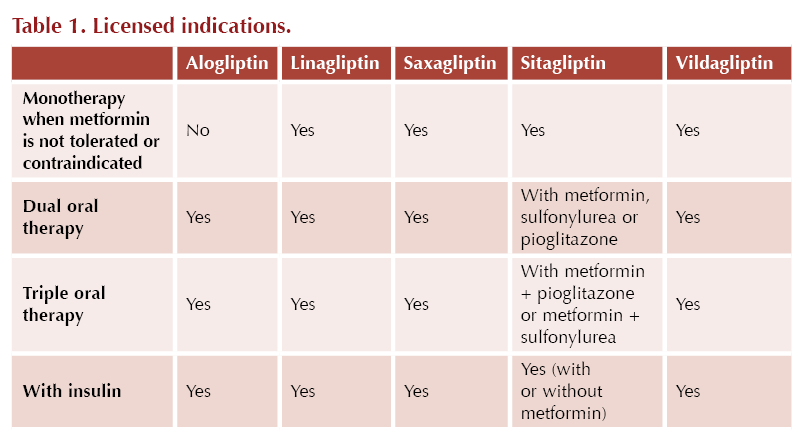
Positioning in guidelines
In the NICE NG28 guideline,3 the DPP-4 inhibitors may be used first-line when metformin is contraindicated or not tolerated. DPP-4 inhibitors can also be used in dual therapy or in triple therapy with metformin when blood glucose is not adequately controlled to individualised targets.
The ADA/EASD consensus report4 highlights the use of DDP-4 inhibitors when intermediate efficacy for glucose lowering is required and where weight management goals would prefer a weight-neutral approach. DPP-4 inhibitors are not recommended for cardiorenal risk reduction in high-risk individuals.
Glycaemic effects
The DPP-4 inhibitors appear to have similar in-class glycaemic efficacy. They result in modest improvement in HbA1c, with a reduction of around 0.5–1.0% when used as monotherapy and around 0.6–1.1% when used in combination with metformin, depending on the agent, dose of therapy and starting HbA1c.2,5
DPP-4 inhibitors reduce post-prandial glucose excursions by around 3 mmol/L and basal glycaemia by around 1.0–1.5 mmol/L.
Effects on body weight
DPP-4 inhibitors are weight-neutral, and thus they may be an attractive option for some patients if other options would likely cause additional weight gain.
Cardiovascular safety
DPP-4 inhibitors provide no benefits above the standard of care for people with established or high risk of atherosclerotic cardiovascular disease, heart failure or chronic kidney disease; however, they have mostly demonstrated cardiovascular safety.
In the SAVOR TIMI 53 trial, saxagliptin had no significant difference compared to placebo in the rate of ischemic events; however, the rate of hospitalisation for heart failure was increased.6
In the EXAMINE trial, a safety signal for increased risk of hospitalisation for heart failure was identified with alogliptin, particularly in people with a history of heart failure.7
A meta-analysis of outcomes data from completed cardiovascular outcome trials suggests that, apart from saxagliptin, DPP-4 inhibitors have a neutral effect on the risk of cardiovascular death, myocardial infarction, stroke and hospitalisation for heart failure.8
Side effects
Common: Headache, cough, nasopharyngitis, abdominal pain, dizziness, fatigue, increased infection risk, gastro-oesophageal reflux, skin reactions, vomiting.
Uncommon: Constipation, skin reactions, arthralgia, hypoglycaemia, peripheral oedema.
Frequency unknown: Angioedema, back pain, cutaneous vasculitis, interstitial lung disease, joint disorders, myalgia, pancreatitis, renal impairment, Stevens–Johnson syndrome, hepatic function abnormalities.
Contraindications
● Any sign of acute pancreatitis (e.g. persistent, severe abdominal pain)
● Hypersensitivity to DPP-4 inhibitors in the past
● Pregnancy/breastfeeding
● Some DPP-4 inhibitors are contraindicated at certain thresholds of renal impairment (see Table 2)
● Some DPP-4 inhibitors are contraindicated in hepatic impairment (see Table 3)
● Type 1 diabetes
● Diabetic ketoacidosis
Cautions
● History of pancreatitis
● Moderate heart failure (saxagliptin only, due to limited experience)
● Severe heart failure (saxagliptin and vildagliptin, due to limited experience)
● Some DPP-4 inhibitors are cautioned at certain thresholds of renal impairment, and dose reductions may be required (see Table 2)

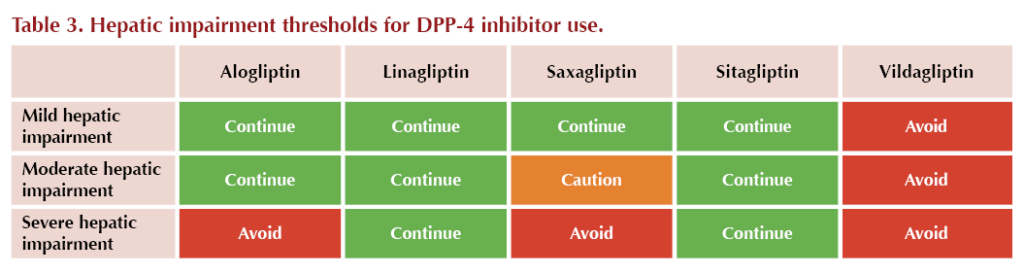
Drug interactions
No significant interactions have been reported between DPP-4 inhibitors and any other drugs. The gliptins do not significantly modify the pharmacokinetic profile and exposure of other medicines, and vice versa. Therefore, no dosage adjustment is usually recommended when gliptins are combined with other pharmacological agents. The notable exception to this is saxagliptin.
Saxagliptin is metabolised to an active metabolite by CYP3A4/5 enzymes.9 Exposure to saxagliptin and its primary metabolite is reported to be significantly altered when saxagliptin is co-administered with specific strong inhibitors (e.g. ketoconazole, diltiazem) or inducers (e.g. rifampicin, dexamethasone) of CYP3A4/5. Glycaemic control should be carefully assessed when saxagliptin is used concomitantly with a potent CYP3A4/5 inducer.
There is an additive effect of using a DPP-4 inhibitor alongside other agents that decrease blood glucose; thus, a dose reduction of agents that act independently of glucose levels (e.g. sulfonylureas) may be indicated to avoid hypoglycaemia.9
Use of DPP4-inhibitors in older frail populations
Oral DPP-4 inhibitors have few notable side effects and are associated with a minimal risk of hypoglycaemia. DPP-4 inhibitors have been formally evaluated in frail older adults, demonstrating efficacy and safety profiles similar to those in younger adults.
DPP-4 inhibitors are one of the few oral agents that are useful in patients with significant renal impairment.
Caution should be taken with saxagliptin in adults with a history of heart failure or in those at risk of heart failure.6 Care should also be taken with alogliptin.7
Initiating and monitoring
If adding a DPP-4 inhibitor to sulfonylurea, meglitinide or insulin therapy, consider whether a dose reduction of the sulfonylurea/meglitinide/insulin is needed to reduce hypoglycaemia risk.
Prior to initiating saxagliptin, vildagliptin or alogliptin:
- Check liver and kidney function
During treatment:
- Vildagliptin: Monitor liver function tests (LFTs) at 3-monthly intervals post-initiation for the first year and periodically thereafter. If transaminase levels increase, repeat the LFTs and monitor until these normalise.
- Saxagliptin: Monitor renal function periodically.
- Alogliptin: Monitor renal function periodically.






Benefits of SGLT2 inhibitors on CKD progression and hospitalisation for heart failure are similar regardless of diabetes status, uACR or eGFR levels.
24 Nov 2025