Case presentation
Pam, a 47-year-old Caucasian lady, attended surgery with symptoms of increased micturition, weight loss and feeling tired. She did not report dysuria or abdominal pain. There had been no change in appetite, no vomiting, no change in bowel habit or passage of loose or fatty stool, and no change in menstrual regularity.
● Past medical history: Two children; nothing else of note.
● Medication: Nil.
● Social history: Married; works in IT sector; non-smoker; 10 units of alcohol per week.
● Family history: None of note.
● Examination: BMI 22.4 kg/m2 (previously 23.1 kg/m2). No abdominal tenderness.
● Investigations: Glucose +++ on dipstick urine, nil else. Fingerprick glucose 17.6 mmol/L, ketones not significantly raised.
Do you have any particular concerns over Pam’s clinical presentation? What would be your next steps in managing the case?
Case analysis
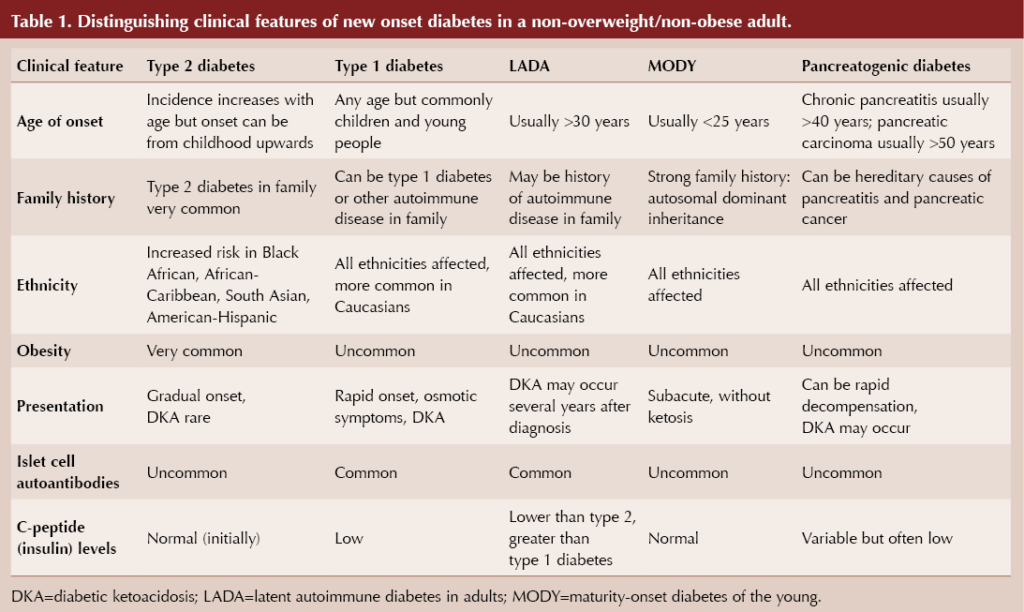
The diagnosis of diabetes needs to be confirmed with laboratory measurement, but then the key question is what type of diabetes does Pam have. Careful consideration of the underlying cause of new-onset diabetes is required in adults who are not overweight or obese. Possible causes are listed in Box 1, with distinguishing features in Table 1 (Morris and Morris, 2024).

Differential diagnosis
First, consider the possibility of type 1 diabetes because decompensation to diabetic ketoacidosis (DKA) is a life-threatening situation. The absence of blood ketones in Pam’s case excludes DKA, although the possibility of an autoimmune diabetes associated with pancreatic beta-cell autoantibodies remains.
Although Pam has no personal or family history of autoimmune disease, her age and normal BMI make latent autoimmune disease in adults (LADA) an important consideration. Best considered as a slowly developing form of type 1 diabetes, LADA does run the risk of decompensation to DKA even if the absolute requirement for insulin typically occurs several years after diagnosis (Pieralice and Pozzilli, 2018).
Maturity-onset diabetes of the young (MODY) is inherited in an autosomal dominant fashion, and Pam’s lack of family history of diabetes is a strong argument against this diagnosis, which furthermore usually presents below the age of 30 years (Urakami, 2019).
Pam has no symptoms suggesting chronic pancreatitis or previous acute pancreatitis. Other causes of pancreatogenic diabetes are less common, including pancreatic cancer, which is a very rare diagnosis in people under the age of 50 years (Duggan and Conlon, 2017).
Beyond these diagnoses, non-overweight/non-obese type 2 diabetes remains a possibility.
Case outcome
Pam was referred urgently to hospital diabetes clinic as having atypical type 2 diabetes, with a concern that she may have had LADA.
Screening for pancreatic beta-cell autoantibodies (GAD, IA2 and ZnT8 autoantibodies) was negative, effectively excluding autoimmune diabetes (type 1 diabetes and LADA). CT scan of the pancreas did not show any abnormality. Faecal elastase levels, which would be low in chronic pancreatitis because of pancreatic exocrine deficiency, were normal. These latter two findings ruled out pancreatogenic diabetes. C-peptide levels were within normal range, indicating adequate insulin reserve and consistent with type 2 diabetes.
By excluding other forms of diabetes, it was concluded that Pam was likely to have type 2 diabetes despite not being overweight or obese.
Type 2 diabetes and BMI
The classic phenotype of an individual with type 2 diabetes in Western society is that of overweight and obesity predisposing to insulin resistance. Insulin production may initially increase in an attempt to overcome this elevated insulin resistance, but when this coping mechanism fails, a relative deficiency of insulin secretion arises and hyperglycaemia ensues (Salvatore et al, 2022). This situation contrasts with the absolute insulin deficiency that is responsible for type 1 diabetes.
The risk of type 2 diabetes increases significantly with people who are overweight (BMI 25.0–29.9 kg/m2) and even more dramatically in those with obesity (BMI >30 kg/m2). However, when other forms of diabetes are excluded, there remains a significant population with type 2 diabetes who have a low or normal BMI (American Diabetes Association, 2024). In fact, around 10% of people classified as having type 2 diabetes in England are not overweight or obese (Public Health England, 2014).
It is well established that people with an Asian, Chinese, African or African–Caribbean family background are more likely to develop type 2 diabetes at a lower BMI than white populations (Chiu et al, 2011). Thus, in a UK Biobank study for example, Asian Indians carried an equivalent risk of type 2 diabetes at a BMI of 22 kg/m2 as Caucasians with a BMI of 30 kg/m2 (Ntuk et al, 2014).
When assessing overweight and obesity in Asian, Chinese, African or African–Caribbean populations, the following lower BMI thresholds have been advised (NICE, 2023):
- Overweight: BMI 23.0–27.4 kg/m2.
- Obesity: 27.5 kg/m2 or above.
The global epidemic of type 2 diabetes most evident in the large populations of India and China will include substantial numbers of people who are not overweight or obese according to their BMI measurement (Salvatore et al, 2022). In Western society, this phenotype will generally be concentrated in ethnic minorities.
Central adiposity and insulin resistance
Central adiposity arises when excess fat is deposited intra-abdominally, rather than subcutaneously. It is the accumulation of this visceral fat in and around the liver and pancreas, as well as ectopic fat deposition in skeletal muscle, that is the key factor driving insulin resistance and the development of type 2 diabetes (Chan et al, 1994). Fat accumulation within the liver results in decreased peripheral glucose uptake and increased hepatic glucose output from the liver.
BMI does not take account of lean versus fat body mass, nor does it separate the fat component into subcutaneous or visceral, making it a poor surrogate marker for insulin resistance (NICE, 2023). People with Asian, Chinese or African ethnic origin tend to have proportionately higher degrees of visceral adiposity, and it is this parameter, rather than BMI per se, that dictates their risk of insulin resistance, type 2 diabetes and cardiometabolic problems.
Waist-to-height ratio
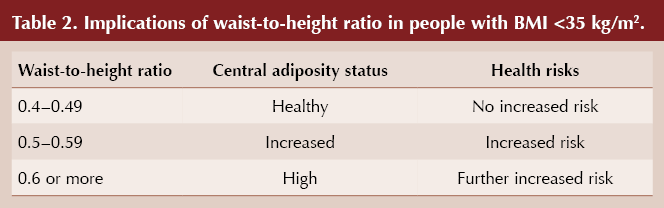
A more accurate measure of central adiposity, and hence risk of type 2 diabetes and cardiovascular disease, is waist circumference (Janssen et al, 2004). NICE (2023) recommends measuring waist-to-height ratio in people with a BMI under 35 kg/m2. Table 2 outlines the implications of these measurements, which are applicable to all ethnicities.
Reduced insulin secretion in people with non-overweight/non-obese type 2 diabetes
A second pathophysiological mechanism operating in non-overweight/non-obese people with type 2 diabetes is impaired insulin secretion secondary to reduced beta-cell function. With central adiposity, fat accumulation in the pancreas not only increases insulin resistance but can also lead to loss of beta-cells and reduced beta-cell function (Ou et al, 2013; Guglielmi and Sbraccia, 2018).
Decreased insulin secretion arising from a reduced beta-cell mass can be the result of intrauterine growth retardation or early post-natal malnutrition and, more specifically, low dietary protein (Gujral and Narayan, 2019; Garg and Dutta, 2019).
Low BMI and malnutrition-related diabetes
The term “malnutrition-related diabetes” has previously been used to describe people, typically in developing countries, with a low BMI (<18.5 kg/m2) who have non-insulin-dependent diabetes (Hugh-Jones, 1955; George et al, 2015). In addition to compromised insulin production from reduced beta-cell mass, insulin resistance arising from sarcopenia (these individuals suffer from severe muscle wasting) may be an important mechanism (Vaag et al, 2006).
In such individuals, insulin production remains sufficient to prevent ketosis but is reduced to the extent that even small degrees of insulin resistance cannot be adequately compensated for, and thus hyperglycaemia ensues (Salvatore et al, 2022). A recent study sought to further characterise this group of people with diabetes and low BMI and concluded that they had a unique metabolic profile, reviving the idea of a distinct diabetes class (rather than considering it as a low-BMI variant of type 2 diabetes) (Lontchi-Yimagou et al, 2022).
Personal fat thresholds
The concept of a personal fat threshold for subcutaneous fat storage, independent of BMI, which if exceeded leads to visceral fat deposition and predisposes to type 2 diabetes, has been proposed (Taylor and Holman, 2015).
In the ReTUNE study, conducted in people with type 2 diabetes who had a normal or near-normal BMI (<27 kg/m2), 70% of participants achieved sustained remission of their type 2 diabetes by means of weight loss induced by a very-low-calorie total-diet-replacement regimen, with an average weight loss of 6.5% (Taylor et al, 2023). The key mechanisms were reduction of intrahepatic fat and recovery of beta-cell function. The conclusion from this study was that loss of “excess weight” in people with normal or near-normal BMI could achieve type 2 diabetes remission in a similar fashion to people with obesity.
Management of non-overweight/non-obese type 2 diabetes
There are no specific guidelines for managing non-overweight/non-obese type 2 diabetes. The evidence would indicate the need to screen for type 2 diabetes at lower BMI thresholds in minority ethnic groups. A positive family history of diabetes in a person in a minority ethnic group is a powerful risk factor irrespective of BMI.
It is well established that weight loss in non-obese people with type 2 diabetes leads to improved blood glucose levels (UKPDS Group, 1990). Losing weight reduces central adiposity and lowers insulin resistance, notably from the loss of fat from the liver (Seppälä-Lindroos et al, 2002). Therefore, advice on diet and physical exercise, with weight loss as a target, should be offered.
Weight loss also reduces fatty pancreatic disease, which can promote recovery of beta-cell function and thus improve insulin production (Taylor et al, 2018). Avoidance of smoking and excess alcohol is of great significance because, in addition to reducing cardiovascular risk, it can help preserve beta-cell function (George et al, 2015). Pharmacological treatments that preserve beta-cell function may have a key role in treatment (Gujral Narayan, 2019).
Metformin is effective in improving glycaemic control in non-obese people with type 2 diabetes and so remains a first-line pharmacological treatment option (Ito et al, 2010). The choice of subsequent agents will be individualised based on comorbidities, need to avoid hypoglycaemia and the importance of weight loss (NICE, 2022).
People with non-overweight/non-obese type 2 diabetes are at high risk of cardiovascular events, and it is essential that treatment of hypertension and use of statins for dyslipidaemia are rigorously addressed.





Poster abstract submissions are invited for the 21st National Conference of the PCDO Society, which will be held on 19 and 20 November.
10 Apr 2025