Update history: Updated 26 March 2024 to include more detailed referencing.
What are HCL systems and how do they work?
An HCL system, also known as an “artificial pancreas” or Automated Insulin Delivery (AID) system, refers to the use of an insulin pump that is “looped” with an algorithm that continuously takes data from a continuous glucose monitoring (CGM) device to make and communicate decisions on insulin dosing to maintain glucose levels within the target range.
There are three elements to the HCL system:
1. A real-time CGM device.
2. An insulin pump that houses a reservoir for rapid-acting insulin.
3. A computerised algorithm, held either on the pump itself or on a smartphone app, that continuously uses data from the CGM device to regulate insulin delivery via the pump.
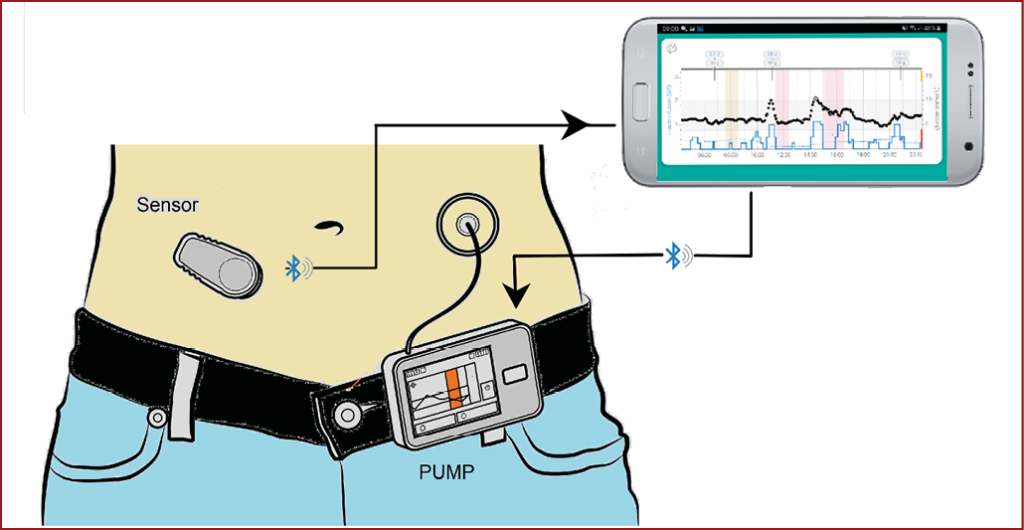
CGM devices for HCL systems
The CGM device measures interstitial glucose every 1–5 minutes (see How to initiate and support continuous glucose monitoring)2 and transmits data by Bluetooth to the insulin management algorithm on either the pump or the smartphone. The algorithm autoregulates pulsed insulin delivery from the insulin pump, constantly adjusting in response to CGM data.
The range of CGM devices used in HCL systems is different to and broader than those available on an FP10 prescription in primary care. Usually, the CGM will be supplied from specialist centres. These CGM devices are the Guardian 4 (also known as Medtronic G4), Dexcom G6, Dexcom G7 and FreeStyle Libre 3. Details can be found in the DSN Forum CGM Comparison Chart here.3
Insulin pumps
There are a variety of insulin pumps available. All contain a reservoir of rapid-acting insulin that can be delivered both continuously in a steady “basal” state and by “bolus” when required around meal times and snacks. All pumps have apps or controls that allow the user to adjust insulin delivery when needed.
Broadly, there are two different types of pumps:
1. Patch (or tubeless) pumps: These are small, disposable devices with an in-built cannula. They can be worn on the upper arm, lower abdomen or upper outer thigh and require changing every three days. Examples include the Omnipod 5.
2. Tethered insulin pumps: These can be attached to a belt or kept in a pocket and deliver insulin via a short infusion set. Both the infusion cannula and insulin reservoir require changing every few days. Examples include the Medtronic 780G, the Tandem t:slim X2, the YpsoPump and the Dana i.
HCL systems used in the NHS
There are a range of different HCL control algorithms available in the NHS. These include Medtronic SmartGuard, Tandem Control IQ, CamAPS FX and Insulet SmartAdjust.
Only one system, the CamAPS FX, is approved for use in pregnancy.
A DSN Forum Comparison Chart for the HCL systems can be found here.4
Functionality of HCL systems
Note that HCLs are not fully automated systems; users still need to embrace the technology and work with it, as there is need for active management. Every time users eat or drink, they will need to tell the system how much carbohydrate they consumed. Users also need to change the settings on the system approximately 1 hour prior to exercise, to protect them from hypoglycaemia during activity.
The whole system requires regular maintenance, with cannula and insulin reservoir changes every 2–3 days. Despite this, for many users, access to insulin pump or HCL technology reduces diabetes burden and can be life-transforming.
Evidence for use of HCL
There is high-quality evidence that HCL technology results in better glycaemic control than insulin pump or sensor-augmented pump therapy, achieving more time in target glycaemic range, less time in hyper- and hypoglycaemia, less severe hypoglycaemia and improved HbA1c.5–7 In pregnancy, HCL leads to substantial improvements in glucose control, which will translate into improved outcomes for both mother and baby.8 Most importantly, HCL is associated with improved quality of life; generally positive user experience, including reassurance and reduced anxiety; improved sleep and confidence; and a reduction in the demands of living with type 1 diabetes.
However, it is important to recognise that benefits may be counterbalanced by important challenges, such as variable levels of trust in technologies; concerns about the physical size or bulk of devices; technical glitches that require troubleshooting to ensure safety; and the challenges associated with incorporating HCL into everyday life.9

Who is eligible for HCL technology?
NICE TA943 guidance1 recommends HCL systems as an option for managing blood glucose levels in:
1. Children and young people with type 1 diabetes.
2. People with type 1 diabetes who are pregnant or planning pregnancy.
3. Adults with type 1 diabetes who have HbA1c ≥58 mmol/mol (7.5%) or disabling hypoglycaemia despite best possible management with at least one of the following:
- Continuous subcutaneous insulin infusion (CSII).
- Real-time CGM.
- Intermittently scanned CGM.
However, not everyone will be able to access HCL technology immediately. Access will be rolled out over 5 years, and the guidance recommends prioritising children and young people, those who are pregnant or planning pregnancy, and those who are already on an insulin pump.
The guidance also highlights the requirement for a cost-effective pricing structure between the technology companies and NHS England.
Equitable access
One third of people living with type 1 diabetes do not access specialist services. Not attending a specialist service is associated with older age, minority ethnicity and social deprivation. Not attending specialist care is a barrier to accessing various diabetes technologies, including CGM, insulin pumps and HCL, as these have typically been initiated in specialist care.
In 2022, the updated NICE NG17 guideline recommended CGM for all people living with type 1 diabetes.10 This should be made available regardless of whether the person chooses to attend primary or secondary care. To ensure your local population will be eligible for HCL in line with NICE TA943, a first step is exploring whether those attending your practice with type 1 diabetes already have access to CGM. A second consideration, if they meet the criteria above, is exploring whether they would like referral to the specialist service for more detailed discussions about what HCL might be able to offer them.
What else do primary care clinicians need to know about HCL systems?
Management of hypoglycaemia
Advice regarding the management of hypoglycaemia needs to be tailored for HCL users. The automated feedback loop between CGM and pump insulin delivery means that, in the event of falling glucose levels or impending hypoglycaemia, insulin delivery is slowed or suspended, helping to avoid hypoglycaemia or limit its severity. The half-life of rapid-acting insulin is very short and, once suspended, insulin is cleared very quickly, allowing the glucose level to stabilise rapidly and then to rise again.
Consequently, self-management of hypoglycaemia in the context of HCL systems requires different advice. HCL users require approximately half the usual recommended glucose dose when treating hypoglycaemia. As a rule of thumb, 5–10 g of carbohydrates should suffice (e.g. 1–2 jelly babies).
Unexplained hyperglycaemia and sick day rules
Hyperglycaemia should be a rare occurrence for HCL users. However, when this does happen, it can suggest a problem with the cannula or infusion set or, less commonly, pump failure. Glucose levels unexpectedly rising above 15 mmol/L and not correcting within 2 hours should raise concern about interrupted insulin delivery. Users should assume the pump may not be delivering insulin and take action:
- Check ketones.
- Give a correction bolus from a pen.
- Change the pump (patch pump users) or infusion set (tethered pump users).
- “If in doubt, change it out!”
A guide for the management of unexplained hyperglycaemia and sick day rules can be found in the Figure below.
Pump insulin delivery failure is uncommon. However, where it does occur, there is danger of rapidly rising glucose and ketone levels, which can lead to diabetic ketoacidosis. Because of this, all insulin pump and HCL users must have access to in-date insulin pens and needles, and the specialist team should have given them information on how much insulin to administer in the event of system failure. Backup supplies should be carried by the person with diabetes at all times for use in the event of unexplained, unresolving hyperglycaemia. In addition, all people with type 1 diabetes should have access to in-date blood glucose and blood ketone testing strips and meters.

Essential supplies on repeat prescription for those on HCL
- Rapid-acting insulin vials for the pump.
- Insulin pens (both long-acting and rapid-acting insulin) and needles.
- Blood ketone strips.
- Blood glucose strips.
- Ensure the person has an in-date glucagon injection kit.
- DO NOT remove insulin pen devices, glucose testing strips or ketone testing strips from the repeat prescription.
Driving and the DVLA
People using HCL systems should follow the same DVLA regulations as the ones applied to all people with type 1 diabetes.
See How to assess fitness to drive.11
Conclusion
The NICE TA943 recommendations have the potential to substantially change the management and improve the outcomes of people living with type 1 diabetes across England. Although these changes will be implemented gradually over the next five years, it is important that all clinicians interacting with people with diabetes have an awareness of the technology. In particular, supporting people with diabetes to have ongoing access to the equipment they need to safely manage and troubleshoot when using HCL will be important.
Where to find out more
For those keen to learn more about HCL systems, we recommend the following resources:
- DTN best practice guide for HCL systems12
- International Consensus for the Use of Automated Insulin Delivery technologies in Clinical Practice13
Disclosures
Emma Wilmot has received personal fees from the Association of British Clinical Diabetologists, Abbott, AstraZeneca, Dexcom, Eli Lilly, Glooko, Insulet, Novo Nordisk, Sanofi and Ypsomed. She has received research support from Abbott, Embecta, Insulet, Novo Nordisk and Sanofi.





3-point MACE occurred in 12.2% vs 13.1% of tirzepatide and dulaglutide recipients, respectively, confirming non-inferiority.
19 Jan 2026