What is gastroparesis?
Gastroparesis can be defined as an abnormal delay to gastric emptying, in the absence of mechanical obstruction. While it has multiple aetiologies, it is commonly associated with diabetes (29% of cases), post-gastric surgery (13%), Parkinson’s disease (7.5%) and collagen vascular disorders (4.5%).1 It is idiopathic in around a third of cases.
Diabetic gastroparesis is a form of diabetic autonomic neuropathy.
Pathophysiology
The underlying pathological cause of gastroparesis has not been fully elucidated; however, it is known to involve the loss of the interstitial cells of Cajal. These cells, which are distributed through the stomach and small intestine walls, trigger electrical activity in the stomach musculature and are often known as the pacemaker of the stomach.
Other factors involved in the development of gastroparesis include diabetes-related neuropathy of the parasympathetic nerves that innervate the stomach, reduction in nitric oxide synthase, angiopathy and altered macrophage activity in the stomach musculature. Additionally, high blood glucose levels, as well as promoting direct slowing of gastric emptying, may lead to many of the neuropathic features described here when chronically elevated.
Identifying and diagnosing gastroparesis
Gastroparesis is likely under-reported; the DCCT-EDIC study identified between 20% and 50% of participants with type 1 diabetes affected.2 It was also noted in up to 30% of people with long-standing type 2 diabetes3, often (but not always) in association with other diabetes complications such as retinopathy, neuropathy and nephropathy.
Gastroparesis is more common in women and increases in prevalence with age.4
People with diabetic gastroparesis may report several symptoms, the most common being:
- Nausea.
- Vomiting.
- Bloating.
- Early satiety.
- Abdominal pain.
- Dyspepsia and reflux.
- The symptoms may result in poor appetite and weight loss.
Some people with diabetes may not have very prominent symptoms, whilst others seem to have pronounced symptoms. There is poor correlation between symptoms and evidence of abnormal gastric motility.
Investigations
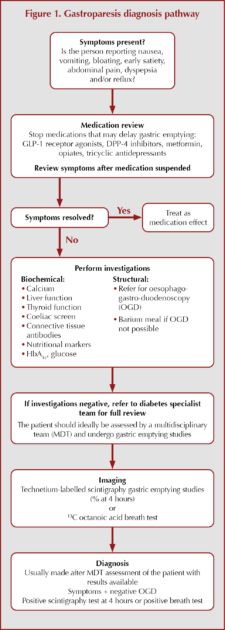
A general pathway to diagnosing gastroparesis is outlined in Figure 1.
When considering gastroparesis in a person with diabetes, it is worth exploring the symptoms they present with. If symptoms are present, it is important to review medications and assess again after stopping drugs that may cause delayed gastric emptying. Several treatments used for the management of type 2 diabetes (e.g. metformin, DPP-4 inhibitors, GLP-1 receptor agonists) and other conditions (e.g. opiates and tricyclic antidepressants) may result in gastrointestinal side effects, and it is important to review their use in people with symptoms of gastroparesis.
If changing medication does not improve the symptoms, then undertake baseline biochemical investigations and consider referral for oesophago-gastro-duodenoscopy (OGD) to rule out structural causes.
If all these investigations are negative, refer to diabetes specialist teams who can, ideally in the setting of a multidisciplinary team, proceed to further investigations and a management plan.
Support and signposting
Gastroparesis is a complex condition often requiring the help of many different medical specialities. This is a difficult journey for the person, with some needing significant nutritional support, others needing support for pain and many struggling to cope with the significant symptoms alongside diabetes control. Some may require psychological support. Above all, the team should be understanding and help steer patients to the best supportive care.
- Basic information from www.nhs.uk is available here.
- Diabetes UK offers advice and support here.
- A good factsheet can be found at Guts UK here.
Management of gastroparesis
Once the diagnosis has been made, a structured multidisciplinary management plan needs to be agreed with the person. This can involve specialists in diabetes care, dietitians and gastroenterologists. Some people will also benefit from psychological support and/or pain management.
Nutritional management
Ideally, a dietitian experienced in diabetes and gastroparesis would work with the individual to agree a plan. The aim is to alleviate symptoms and enhance glycaemic control. Various dietary options may be considered. A trial of low-fat, low-fibre, small-particle or liquid diets may be beneficial.5,6
As most people with gastroparesis (around 60%) consume fewer calories than is recommended, ensuring good nutrition is very important; some people may require nutritional supplements. In some cases, enteral feeding may be required for relief of symptoms and/or to meet ongoing nutritional needs.
Diabetes management
Controlling short-term glycaemic variability and long-term glucose management is important, both to limit the effect of glucose on gastric emptying and also to reduce the risk of further complications. Various strategies may need to be considered.
Insulin timing or splitting doses of insulin may need to be considered for those who are on insulin therapy. For some people with type 1 diabetes, insulin pump or hybrid closed-loop therapy may be required, and this will require support from a diabetes specialist team.
Gastroenterology management
Ruling out structural lesions causing delayed gastric emptying is the first step in making a diagnosis of gastroparesis. Additionally, further interventions can be made to improve symptoms in certain circumstances – see treatment pathways.
Medical therapy
Various pharmacological strategies are available to improve the symptoms of gastroparesis; treatment should aim for relief of the most prominent or bothersome symptoms.
Dopamine receptor antagonists
These predominantly include drugs such as metoclopramide or domperidone, both of which have been shown to increase gastric emptying7 and are also potent anti-emetics. However, metoclopramide has been associated with dystonias such as an oculogyric crisis and, rarely, tardive dykinesia.8 Long-term use can increase the risk of developing Parkinsonism. Domperidone has been associated with prolongation of QT interval and is relatively contraindicated in people with known cardiac disease.9 The MHRA/CHM do not recommend long-term use of metoclopramide and caution with domperidone.
- Metoclopramide: 10 mg three times daily orally, although liquid preparations are more effective. Should be reserved for short-term use in hospital settings.
- Domperidone: 10 mg twice to three times daily orally. Use for symptomatic relief for the shortest time possible.
Motilin receptor agonists
The macrolide antibiotics erythromycin and azithromycin have prokinetic effects in the stomach and the small bowel.
Erythromycin is effective as a prokinetic at a dose of 50–100 mg three times a day.10 It is useful in the short term but is associated with tachyphylaxis and thus not suitable for long-term use. Side effects include disturbance to bowel habit and possible cardiac side effects.
Other anti-emetics
Standard anti-emetics can help with nausea but generally not with other symptoms. If, however, nausea is the most prominent symptom, they can help. Medications such as cyclizine or ondansetron are useful here.
Interventional therapies
Procedures such as pyloromyotomy, known as G-POEM (gastric peroral endoscopic myotomy) when performed endoscopically, have been shown to improve gastric emptying, and some data suggest correlation with improved symptoms.11
Surgical pyloroplasty, although more interventional, has also been shown to provide relief from some of the symptoms of gastroparesis.4
Interventions such botulinum toxin injections in the pylorus, despite showing promise initially, have failed to demonstrate ongoing effectiveness and are no longer recommended.
Other interventions
Occasionally, interventions such as percutaneous gastrostomy or jejunostomy tubes are required for relief of symptoms and/or to meet ongoing nutritional needs. These procedures should be fully discussed in the multidisciplinary team setting with the individual.
Relevant guidelines
Gastroparesis is covered, briefly, in the complications section of the NICE NG17 guideline on the management of type 1 diabetes.
Click here to access
It is also briefly covered in the NICE NG28 guideline on type 2 diabetes management.
Click here to access






Jane Diggle examines the draft update to the NICE NG28 clinical guideline, plus new advice regarding the discontinuation of Levemir.
10 Sep 2025