Case history: The presentation
Emily (a pseudonym), age 68 years, had blood tests performed ahead of her annual hypertension monitoring appointment with her GP. The results were as follows:
HbA1c: <20 mmol/mol
Haemoglobin: 105 g/L
Mean corpuscular volume: 112.3 fL
White cell count: 9.3×109/L
Platelet count: 411×109/L
Sodium: 142 mmol/L
Potassium: 3.5 mmol/L
eGFR: 77 mL/min
TSH: 1 mU/L
Total cholesterol: 2.5 mmol/L; Non-HDL: 1.7 mmol/L; HDL: 0.79 mmol/L
Bilirubin: 27 µmol/L
Other LFTs: Normal range
Urinary ACR: <0.4 mg/mmol
Previous medical history: Hypertension; hyperlipidaemia; hypothyroidism; depression.
Medication: Amlodipine 5 mg once daily, bendroflumethiazide 2.5 mg once daily; atorvastatin 20 mg once daily; levothyroxine 75 µg once daily; citalopram 20 mg once daily.
At review, Emily reported feeling tired but was otherwise asymptomatic. She did not report symptoms suggestive of hypoglycaemia.
On examination, her blood pressure was 132/73 mmHg and her BMI was 28.2 kg/m2.
How would you respond to these results?
Understanding HbA1c
Under normal circumstances, approximately 97% of adult haemoglobin is haemoglobin A (HbA), which itself is composed of four globin chains: two alpha and two beta chains (Wang and Hng, 2021). When glucose binds covalently to the beta chain of HbA, the resulting form of the molecule then persists until the red cell is destroyed. In people without diabetes, around 6% of HbA is glycated, with the principal contribution, typically around 5%, coming from HbA1c (HbA can be glycated at other sites on the molecule). In people with diabetes, who have higher blood glucose concentrations, the level of HbA1c rises.
HbA1c, then, is a measure of the proportion of HbA within red cells that is glycated. It reflects the prevailing blood glucose levels over the lifetime of the red blood cell: approximately 120 days. It is important to note that glucose levels nearer to the time of HbA1c measurement contribute significantly more to the HbA1c value than those more distant from the time of testing, because red blood cells that are more recently glycated will better survive until the time of the test than older red blood cells (Little and Sacks, 2009).
HbA1c thus acts as a surrogate marker for plasma glucose levels over the previous 3 months – an average that is skewed to more recent glucose readings.
Utility of HbA1c in diagnosing and monitoring diabetes
HbA1c is routinely used as a means of diagnosing type 2 diabetes, with a threshold of 48 mmol/mol (6.5%), and is also used as a means of monitoring glycaemic control in all types of diabetes. An important advantage of using HbA1c is that a venous blood sample can be taken at any time, in contrast to fasting plasma glucose and the oral glucose tolerance test (OGTT), both of which require the individual to be fasted and, in the case of the OGTT, detained for a prolonged period. A further benefit of using HbA1c is that it is inherently less subject to the day-to-day variation of other diagnostic tests. HbA1c levels correlate well with diabetes complications.
There are situations, however, when HbA1c should not be used as a diagnostic test for diabetes, notably when glucose levels have risen rapidly (see Box 1). In such situations, HbA1c will not accurately reflect current levels of glycaemia (Farmer, 2012; John, 2012). Remember that HbA1c does not indicate glucose variability, such that two individuals with very different glucose profiles could return the same HbA1c.

Case history: Managing Emily’s presentation
Emily was started on home glucose monitoring, which documented a couple of low glucose episodes (2.8 and 3.6 mmol/L) pre-breakfast, and she reported feeling anxious at those times, with her stomach churning. She was asked to use Lucozade, which did not help her symptoms. At this stage, referral to the endocrinologist was made due to concern about spontaneous hypoglycaemia.
Endocrinologist’s assessment: Absence of typical Whipple’s triad (symptoms of hypoglycaemia, plasma glucose concentration <3.0 mmol/L and resolution of the symptoms after the plasma glucose concentration is raised) suggested that hypoglycaemia was not a significant concern. The presence of anaemia and raised bilirubin indicated the possibility of haemolysis and that this could account for the low HbA1c.
Investigations:
HbA1c: <20 mmol/mol
Fasting plasma glucose: 5.7 mmol/L
Haemoglobin: 109 g/L
Vitamin B12: 364 ng/L
Ferritin: 360 µg/L
Folate: 2.6 µg/L (borderline low)
9 am cortisol (as advised by endocrinology): 340 nmol/L (normal range)
Fructosamine: not analysed (due to laboratory error)
Antinuclear antibodies: positive
ANCA/RF/CCP antibodies: negative
Myeloma screen: negative
Blood film: Anisocytosis, polychromasia, spherocytes, raised reticulocyte count (12.4%)
A direct antiglobulin (Coombs) test was positive
Conclusion: Subsequently, fingerprick glucose readings were all >4 mmol/L, confirming that Emily’s low HbA1c result did not match up with her measured glucose levels. These results strongly suggested the cause of the low HbA1c to be haemolysis, due to an autoimmune haemolytic anaemia. Emily was referred urgently to the haematologist.
Unrepresentative HbA1c results
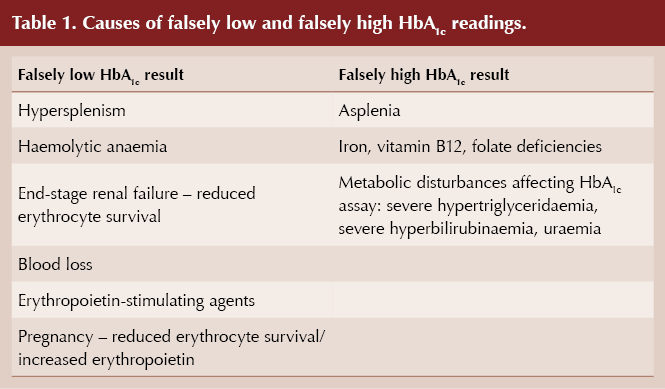
Conditions that reduce the lifespan of red blood cells and trigger increased erythrocyte turnover mean there is less time for glycation of red cells to occur and that HbA1c may, therefore, be falsely lowered (Radin, 2014; Wang and Hng, 2021). In contrast, and unlike in Emily’s case, extended erythrocyte survival or reduced erythrocyte turnover can lead to falsely elevated HbA1c values (see Table 1).
Haemoglobinopathies have a variable effect on HbA1c, and specialist advice should be sought (Radin, 2014). Blood transfusions can falsely lower or raise HbA1c, depending on the diabetes status of donor and recipient. It is also worth being aware that, for given levels of glycaemia, non-Caucasian populations have slightly higher HbA1c values than Caucasians (Herman and Cohen, 2012). HbA1c values should not be relied on in cases of severe anaemia (Hb <100 g/L).
Alternatives to HbA1c for assessing glycaemic control
Clearly, other means of assessing glycaemia need to be utilised in situations where it is suspected HbA1c could be unreliable. As in Emily’s case, self-monitoring of blood glucose (SMBG) can be undertaken, or if necessary some form of continuous glucose monitoring (CGM).
In pregnancy, the 75 g OGTT remains the gold standard for diagnosing diabetes (NICE, 2020). Thereafter, SMBG or CGM can be used to monitor glucose levels and direct treatment.
Serum fructosamine is an alternative measure of glycaemic control in situations where red cell turnover invalidates HbA1c, although it has not been validated as a measure of longer-term glycaemic control or as a predictor of diabetes complications (Rodin, 2014). The test measures glycation of proteins, principally albumin, and reflects glycaemic control over the previous 2–3 weeks, given that the half-life of albumin is around 20 days. However, fructosamine testing would be unreliable in the presence of hypoalbuminaemia.
Conclusion
HbA1c is a pragmatically useful surrogate marker for average glucose control over the preceding 3 months, albeit weighted towards more recent glucose levels. There are clinical situations, however, when HbA1c should not be relied on either to diagnose diabetes or to accurately reflect glucose levels. Unrepresentative HbA1c values can arise through altered erythrocyte lifespan. Under these circumstances, alternative means of assessing glycaemic control should be undertaken.
Further reading
How to correctly diagnose and classify diabetes
A quick guide for primary care on ensuring that diabetes is diagnosed correctly and accurately.
Diabetes & Primary Care 24: 107–10
Click here to access





Findings should motivate us to encourage statin initiation and persistence for primary prevention in more people with type 2 diabetes.
26 Feb 2026