Traditional nutrition therapy aims to achieve normoglycaemia in children with type 1 diabetes by matching insulin to the carbohydrate content of food to mimic normal pancreatic function (Rovner, 2009). This approach implies that a particular size of carbohydrate portion will always have the same effect on blood glucose levels. In real life, however, this does not always result in target glycaemia. Even though we focus on carbohydrate counting, evidence supports postprandial blood glucose levels being affected by both the quality and quantity of carbohydrates, together with the amounts of fat and protein in the meal that, in turn, affect mean blood glucose and HbA1c levels (Kirpitch and Maryniuk, 2011; Bell et al, 2015).
One way of describing the quality of carbohydrates in a food item or meal is to use the concepts of glycaemic index (GI) and glycaemic load (GL):
- GI describes the rate of absorption of food containing carbohydrates (i.e. how quickly blood glucose levels are raised after consumption). Carbohydrate-containing foods are ranked as low (0–55), medium (55–75) or high (>75) GI. The lower the GI rating, the more slowly the carbohydrate is absorbed.
- GL extends the concept of GI to include portion size. It takes into account the quantity of carbohydrates and is expressed as the GI value of the food multiplied by the portion size (g/serving) divided by 100. A low-GL food or meal is classified as <10, while 20 and over is high.
High-GI foods raise blood glucose levels more quickly, resulting in higher postprandial blood glucose levels that may eventually lead to beta-cell failure and insulin resistance in children (Louie et al, 2012). Replacing high-GI foods with a low-GI diet benefits glycaemic management by improving insulin resistance and HbA1c in people with type 1 diabetes as well as those with type 2 diabetes (Brand-Miller et al, 2003). The lower postprandial glycaemic response after eating a low-GI breakfast may be associated with beneficial cognitive and behavioural effects in children, as fluctuations in blood glucose levels are minimised (Benton et al, 2007; Micha et al, 2011; Philippou and Constantinou, 2014). Higher mean blood glucose levels, increased time spent in a higher glycaemic range and high HbA1c are associated with a higher incidence of behavioural problems, such as hyperactivity, aggression and conduct disorders, in primary school children with type 1 diabetes (McDonnell et al, 2007). One study found that a low-GI meal reduced postprandial blood glucose excursion by 30–180 min (as much as 4.2 mmol/L/h) compared to a high-GI meal when preprandial ultra-short-acting insulin was administered (Ryan et al, 2008). A lower-GI/GL meal results in glucose entering the blood at a slower rate that leads to a lesser glycaemic response. Consequently, a lower insulin dose is required as insulin sensitivity is improved without increasing hypoglycaemic events (Quieiroz et al, 2012). This effect of low-GI/GL meals also has important implications for individuals with type 2 diabetes (Kirpitch and Maryniuk, 2011).
Reducing the postprandial curve should be a key focus in achieving treatment-to-target goals, as it is a major contributor to overall HbA1c. It is an essential approach in preventing long-term complications (i.e. cardiovascular diseases and mortality) in type 1 diabetes (O’Connell et al, 2008).
Behaviour change in local children
A few families at The Great Western Hospital had noticed a change in the behaviour of their children with type 1 diabetes and observed the children had difficulty concentrating after eating some types of high-GI breakfast cereals. These changes were accompanied by high blood glucose readings mid-morning and hypoglycaemia at lunchtime in the absence of any corrective doses of insulin. The GI/GL of the breakfast cereal could theoretically explain this, as there is a mismatch between the rate at which insulin acts and glucose is released during this time. Some children might also be experiencing hypoglycaemic events later in the morning before lunch. Even though blood glucose levels might come down without any intervention, there were concerns about the effects higher blood glucose levels were having on children’s learning, behaviour and overall long-term glycaemic outcomes. Ideally, advocating a change in diet, such as promoting lower-GI breakfast cereals, would be the first port of call but it is not always easy to convince children to change eating habits. In addition to this, morning breakfast cereals are a convenient choice on school days when time is limited.
As a result of the behavioural changes observed, it was decided to carry out an assurance project to gain a better understanding of what happens to children’s glucose levels after eating a high-GI/GL breakfast and to assess recommendations that were being given to address postprandial highs in the morning.
Recommendations
Recommendations given included advising that an ultra-short-acting insulin be injected 20 minutes before children ate high-GI/GL breakfast cereals, with the aim of improving post-meal blood glucose highs and avoiding hypoglycaemic episodes. We also advised patients and their families whether any additional insulin was needed if they were going to consume high-GI/GL items for breakfast. This information was provided as a result of the recommendation to increase the insulin-to-carbohydrate ratio for meals with a high GL (Groele et al, 2014).
Method
All children on a multiple daily injection treatment regimen were invited to participate in the project. Participants were aged from 5 to 15 years and had been diagnosed with type 1 diabetes 1–3 years before the start of the project.
Participation provided families with the opportunity to gain practical experience interpreting the Freestyle Libre. Seven families who agreed to participate had a child who had not previously used this glucose monitoring system.
Each child was asked to wear the Freestyle Libre for 2 weeks and during this time to have the same breakfast meal and portion size each day (high GI >75 and GL >20). Participants had to ensure their carbohydrate counting was accurate (i.e. they were required to weigh their cereal and milk) and document everything on a record sheet. Children were asked not to have any snacks between breakfast and lunch unless they had a hypoglycaemic episode, in which case they should treat it as per their individual care plan.
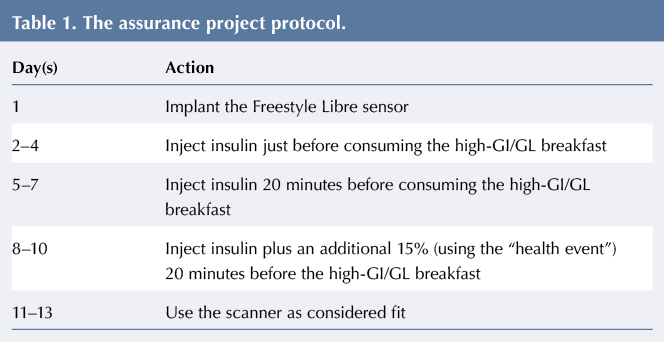
The project protocol is given in Table 1. Children had to inject in their abdomen before breakfast for the duration of the project. A “health event” of +15% was set up on their Accu-Chek Aviva Expert meter to help calculate any additional insulin doses required. They had to scan the Freestyle Libre sensor before breakfast (in addition to carrying out a blood glucose check), 2 hours after breakfast and just before lunch (in addition to a blood glucose check). Participants who had corrective insulin doses at breakfast or experienced hypoglycaemic episodes before or after the meal were excluded from data analysis to avoid confounding the results.
Results
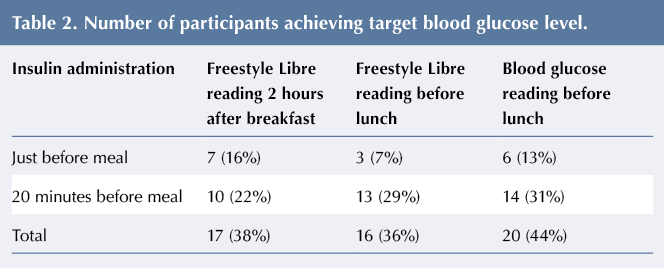
Table 2 gives the number of participants achieving the 2015 NICE-recommended target blood glucose level of 5–9 mmol/L after consuming a high-GI breakfast.
Thirteen per cent of participants achieved target blood glucose before lunch on days 2–4, when injecting insulin just before the meal. On days 5–7, when injecting insulin 20 minutes before the meal, 31% of participants had blood glucose within the target range. On days 8–10, when an additional 15% of insulin was to be administered, the majority of participants required a corrective insulin dose at breakfast due to blood glucose levels >7 mmol/L, and their readings were therefore excluded from the results. The postprandial curves measured by the Freestyle Libre showed lower peak blood glucose levels following the administration of insulin 20 minutes prior to consumption of the high-GI breakfast compared to immediately before consumption (see Figure 1).
The numbers of hypoglycaemic episodes experienced are given in Table 3. One pre-breakfast hypoglycaemic episode was recorded, which was excluded from analysis. Six hypoglycaemic episodes were recorded by the Freestyle Libre 2 hours after breakfast: three when insulin was injected just before the meal and three when insulin was injected 20 minutes before breakfast. Four episodes were recorded before lunch, with 7% of participants experiencing hypoglycaemia following an insulin injection just before the meal and 2% when insulin was injected 20 minutes before breakfast. All of the hypoglycaemic episodes were excluded from the final analysis.
After data from participants experiencing hypoglycaemic episodes and from those requiring insulin dose corrections before or after breakfast were excluded, only data from two participants remained. These data were insufficient for statistical analysis.
Owing to the small number of episodes recorded during the study (n=26; see Table 4), a two-way analysis of variance (ANOVA) showed no significant difference in the number of episodes between groups.
Discussion
The descriptive statistics suggest that injecting ultra-short-acting insulin, such as NovoRapid, 20 minutes before a high-GI/GL breakfast produces a lower 2-hour postprandial curve within the target range without increasing the risk of hypoglycaemic episodes. This finding could be explained by there being a better match between the action of the insulin and the rate of glucose release. This result is promising; however, further research with a larger number of participants is needed to determine whether there is a significant difference in outcomes between insulin given immediately and 20 minutes before the consumption of a high-GI/GL meal. The ANOVA did, however, show a trend. If a power of one had been carried out to show a significant difference, at least 50 episodes would have been required compared to the 26 experienced in this project.
Even though there is significant evidence to support the clinical use and promotion of lower-GI/GL diets in type 1 diabetes and type 2 diabetes, barriers still exist among health professionals relating to the adoption of these dietary principles and recommendations. Barriers identified include the following (Gilbertson et al, 2003; Grant and Wolever, 2011):
- It is too difficult a concept for healthcare professionals to understand and apply.
- It is too difficult to translate to families in practical terms.
- The perception that it will limit food choices and variety while promoting a higher-fat diet.
Although GI might be a complex concept to understand and relate, it is important for healthcare professionals to incorporate these principles into their everyday teachings and recommendations. Developing GI/GL resources is key to breaking down the barriers to applying the principles in practice. Creating diet sheets and resources, see Figure 2, is a small step in the right direction.
Despite this, educational tools and knowledge on GI/GL need to be produced. It is unrealistic to expect families to estimate the GI ranking of each individual food (as many factors influence this); therefore, teaching the general classification of low-, medium- and high-GI foods and meals might be more realistic and should form the basis of education when encouraging people to adapt a meal or swap a food item to a lower-GI/GL option. Instead of focusing too much on the numerical side of GI/GL, which can be fairly demanding, it is recommended that families be encouraged to recognise the importance of healthy eating messages, including eating lean protein, soluble fibre, five fruit and vegetables a day, appropriate types and amounts of fat, and to include a low-GI food in each meal, as recommended by the 2015 NICE guidelines, which support the use of the GI in type 1 diabetes.
There was overwhelmingly positive feedback from families relating to their experiences with the Freestyle Libre. There was only one event of note: a child’s sensor fell out on day 11. All seven of the participants introduced to the Freestyle Libre through the project carried on using it afterwards, as they could see the additional benefit to their diabetes management.
Limitations
Due to the sample size, after excluding results from participants who experienced hypoglycaemic episodes or required a corrective insulin dose meant we were unable to determine whether or not patients should take additional insulin before consuming high-GI/GL meals in order to improve peak postprandial glucose levels and avoid hypoglycaemic episodes. Results showed a trend in favour of injecting insulin 20 minutes before consuming a high-GI/GL breakfast, but again due to the number of participants no significant difference was found. A larger study is needed to gain definitive results.
Conclusion
Children who inject insulin 20 minutes before consuming a high-GI breakfast are more likely to have lower postprandial glucose peaks within the recommended range and less likely to experience hypoglycaemia than those who inject immediately before eating. Further research is needed to build on these results and determine whether additional insulin is required. In the interim, it should be recommended that children with type 1 diabetes wait for 20 minutes after injecting insulin before eating.
Acknowledgement
Thanks to Lucy Rowe, South West Children & Young People’s Diabetes Network Manager, who supported us in this project, and to all the children and families who participated in the study.









NHSEI National Clinical Lead for Diabetes in Children and Young People, Fulya Mehta, outlines the areas of focus for improving paediatric diabetes care.
16 Nov 2022