There is a general trend of increasing childhood obesity that is more pronounced in developed western countries but becoming equally important in developing nations too. The association between obesity and/or overweight with type 2 diabetes (T2D) is well recognised, including in children; the term ‘diabesity’ was coined to describe the presence of diabetes and obesity together. However, the presence of obesity in children with type 1 diabetes (T1D) is less well known or recognised. This article explores the prevalence, evolution, consequences and management challenges associated with diabesity, specifically in children with T1D.
Prevalence and definitions of obesity
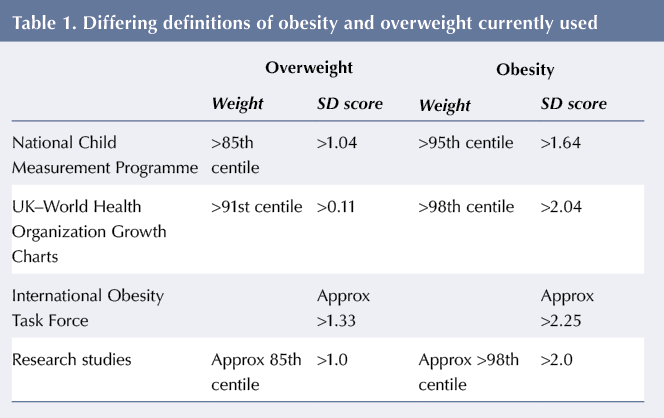
There is a higher prevalence of diabesity in children with T1D, over and above the background prevalence of childhood obesity in the general population (Tomas et al, 2015). However, there are currently different definitions of childhood obesity used in practice, see Table 1, which makes comparison of the available data difficult and less robust.
The National Child Measurement Programme (NCMP) and the United Kingdom–World Health Organization growth charts use different weight centile cutoffs to define overweight and obesity; whereas the International Obesity Task Force and many research studies use body mass index (BMI) standard deviation (SD or Z) scores. According to International Obesity Task Force criteria (Cole and Lobstein, 2012), overweight and obesity are defined as the BMI Z-score corresponding to a BMI >25 and >30 at 18 years of age, respectively, which is equivalent to approximately >2.25 and >1.33 SD scores, respectively, but differs slightly between males and females. Most of the published studies define overweight and obesity as BMI Z-scores above +1 and above +2, respectively. These scores equate to approximately the 85th and 98th centiles on growth charts.
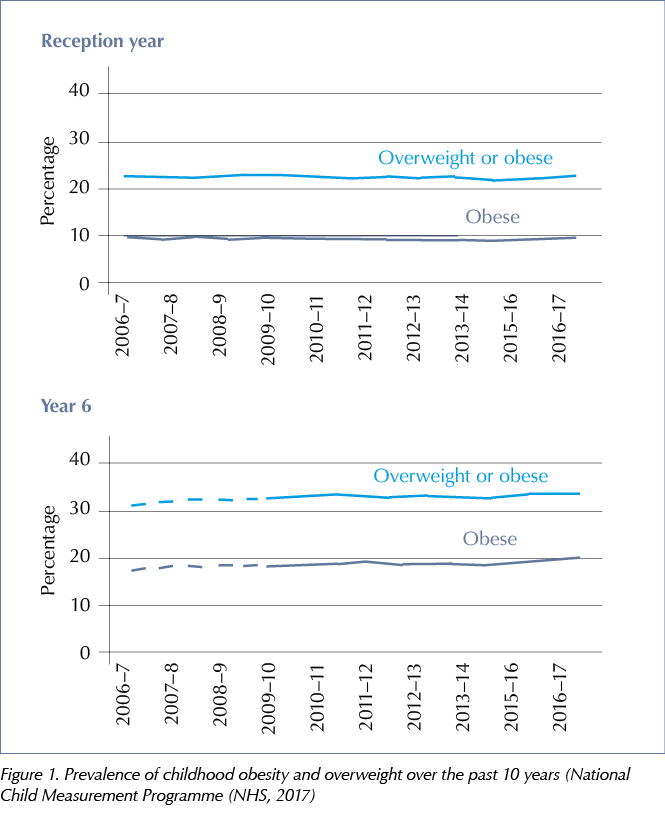
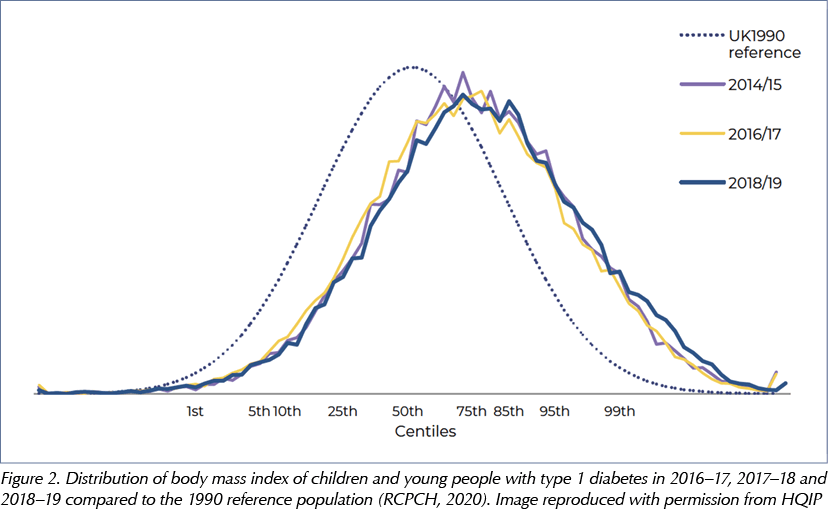
The NCMP currently estimates the prevalence of obesity and overweight in children in England and Wales at two set ages: 5 and 11 years, corresponding to entry (Reception Year) and last year (Year 6) at primary school. The latest data published (from 2016–17, see Figure 1), reported an average prevalence of 10% for obesity and 13% for overweight at primary school entry (NHS, 2017). This prevalence at 5 years has remained static over the previous 10 years from 2007–7 (NHS, 2017). However, by 11 years of age, or the last year at primary school, there was almost doubling in the prevalence of obesity to an average of 20%; and the overweight prevalence levels showed a small increase to 15% (NHS, 2017). These prevalence figures were obtained from the annual National Paediatric Diabetes Audits (NPDAs). The definition used in the NPDA for overweight and obesity corresponds to a weight above the 85th and above 95th centiles, respectively; the same as those definitions used by NCMP. However, one significant difference between the two data sets is that the NPDA obtains the prevalence across ages 1–18 years; whereas NCMP obtains the data at two set ages in childhood. During the 2014–17 period, the NPDA reported an average 20% prevalence of obesity and 18% prevalence of overweight, indicating there has been an increase in the proportion of children with T1D who are overweight when compared to the general childhood population (Royal College of Paediatrics and Child Health [RCPCH], 2016; 2017; 2018). In 2018–19 audit year, the average overweight prevalence remained around 18% (17.7% in 0–11 years and 18.3% in 12–18 years), whereas average obesity prevalence showed a further slight increase to 20.8% (18.1% in 0–11 years and 23.6% in 12–18 years), see Figure 2 (RCPCH, 2020).
The international SWEET Registry collects data from 55 paediatric diabetes centres providing care to over 23,000 children with diabetes; 42 of these centres are located in 25 European Union countries and 13 centres are outside Europe. Overweight and obesity for this international data collection were defined as BMI Z-scores of >+2 and >+1 respectively, using the national growth standards for respective populations. The average cross-sectional prevalence of overweight was 25% (22.3% in boys and 27.2% in girls) and for obesity was 7% (7.3 in boys and 6.8% in girls) across all ages from 1 to 18 years (Maffeis et al, 2018). The reported prevalence of diabesity in this study was lower. This may be due to the different definitions of obesity used by the two datasets and the different populations studied; but the combined obesity and overweight figures were comparable to the NPDA data from England and Wales. Nevertheless, the SWEET Registry shows that across countries the prevalence of combined overweight and obesity in children with T1D was higher than expected in the general childhood population. In addition, it suggests that there are significant associations with duration of diabetes, female gender and poor metabolic control – as evidenced by increased HbA1c – in children with T1D and overweight and/or obesity (Maffeis et al, 2018).
Case report
The evolution of diabesity is highlighted here by an illustrative case of a female patient with T1D.
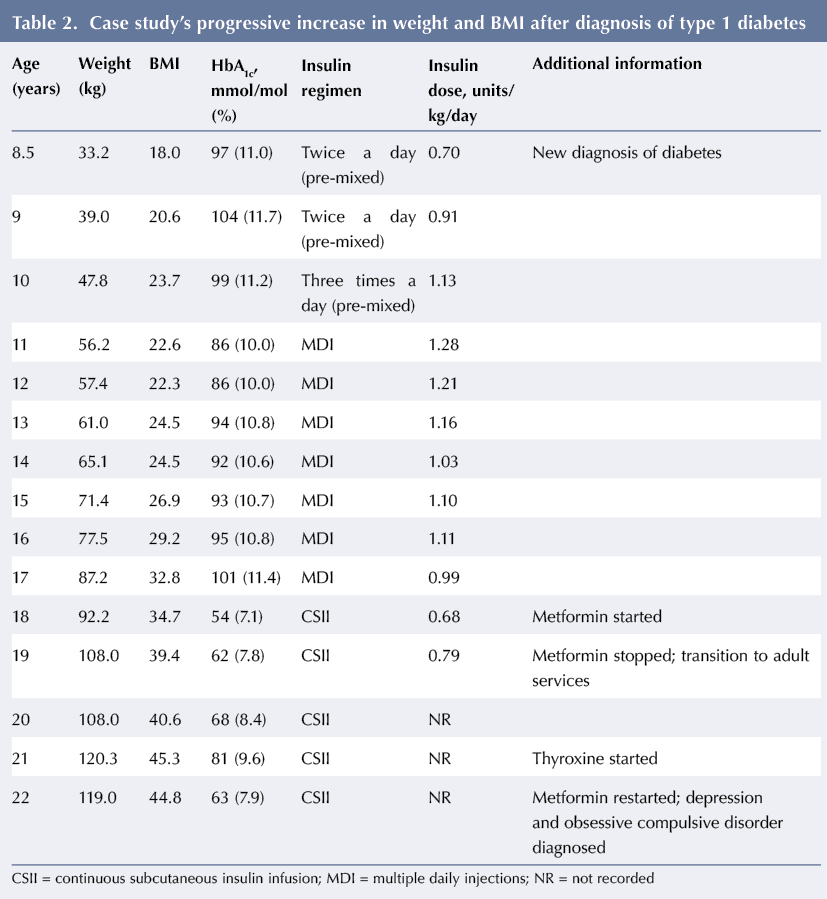
A new diagnosis of T1D was confirmed following a 2-week history of symptoms when the girl was aged 8.5 years. There was a family history of obesity, with one parent (the mother) being moderately obese. The patient’s progress over the next 10 years is summarised in Table 2. During this period, she experienced a gradual and relentless increase in weight and BMI that did not show a beneficial response to metformin therapy. There was associated poor diabetes control, which improved in the short term while she was on intensive insulin pump therapy but this improvement was not sustained in the medium or long term.
At transition, aged 19 years, the young woman had a high BMI of 39.4. She had no significant comorbidities associated with obesity (normal blood pressure, normal kidney function and mildly raised total cholesterol with normal low-density lipoprotein cholesterol); however, her risk of developing comorbidities in the future remains high.
Risk factors for increasing BMI in children with T1D
All risk factors that promote overweight and obesity in the general population also apply to children with T1D, such as an obesogenic environment, sedentary lifestyle, decreased physical activity and consumption of excess calories (Barber, 2016). In addition to these, there are some specific diabetes-associated independent risk factors:
- Age
- Duration of diabetes
- Female sex
- Intensive insulin therapy
- Lower socioeconomic status.
Age
The longitudinal study by De Keukelaere et al (2018) reported that age is an independent risk factor. Starting from age 11–12 years, there is a steep increase in BMI Z-scores; this was steeper for girls compared to boys. Young people with T1D between the ages of 15 and 20 years had the highest BMI Z-scores.
The suggestion of more pronounced weight gain with puberty was not supported by this longitudinal study. Data confirmed the onset of increased weight gain just prior to the onset of puberty (De Keukelaere et al, 2018).
Duration of diabetes
There is evidence in both cross-sectional and longitudinal studies that longer duration of diabetes leads to a progressive increase in BMI Z-scores. The BMI Z-score increased by an average 0.5 after 5 years of diabetes, according to SWEET Registry data (Maffeis et al, 2018). An increase in BMI SD score was also seen with more years since diagnosis in longitudinal assessments. Assuming linearity, the strength of this effect on BMI Z-score increase was estimated at 0.035 per year (De Keukelaere et al, 2018). Plausible explanations for the excessive weight gain could be excessive energy intake due to fear of hypoglycaemia, increased appetite or underestimation of food intake (Kaminsky and Dewey, 2014).
Female sex
The increase in BMI is much more prominent in females than in males, and this is evident both from cross-sectional and longitudinal studies. In the SWEET Registry, HbA1c was poorer and weight gain more prominent in females after 5 years of diabetes duration (Maffeis et al, 2018). In the Belgian longitudinal study, this finding was further clarified. Boys after 10 years of age had an average increase in BMI Z-score of 0.3, whereas in girls the increase was almost double this; but just after 5 years of diabetes duration, there was a more prominent increase in BMI Z-scores in girls (De Keukelaere et al, 2018).
Intensive insulin therapy
There is some evidence that intensive insulin therapy leads to increased weight gain (Apperley and Ng, 2019). This may be due to increased efforts at correction of hyperglycaemia and possibly more hypoglycaemic episodes requiring mandatory sugary food intake. However, this is not confirmed in all studies. In fact, the SWEET Registry data clearly demonstrated that there was no association with conventional or intensive insulin therapy, with pump therapy or multiple daily injections, or with the dose of insulin used in normal weight and overweight children (Maffeis et al, 2018).
Lower socioeconomic status
Ethnicity and other health inequalities are an important factor of unhealthy weight status in the community. This is also true for adolescents with T1D: adolescents in a single-parent household, parental education below a college degree, lack of private health insurance and low household income were all associated with a three- to fourfold increased risk of adiposity in females (Kahkoska et al, 2018). Low household income was associated with a nearly twofold increased risk of adiposity in males (Kahkoska et al, 2018).
Consequences
The consequences of obesity and T1D are inter-linked and it is difficult to differentiate one from the other. In general, obesity influences T1D by increasing insulin resistance, cardiovascular risk profile, and possibly the risk of mental health problems, as well as increasing HbA1c. The presence of acanthosis nigricans in some obese children with diabetes also suggests increased insulin resistance in T1D patients, despite T1D being an insulin deficiency condition.
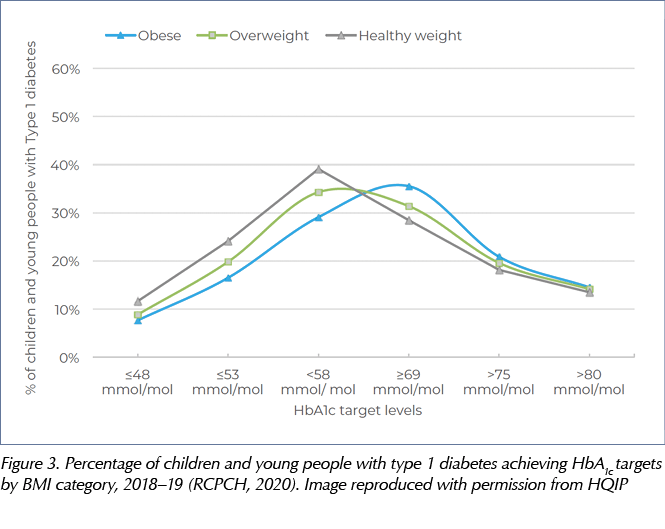
There is a higher prevalence of smoking and higher risk of mental health problems, such as depression and disordered eating, in obese children with diabetes (Prinz et al, 2018). According to the international SWEET Registry and NPDA (see Figure 3), children with T1D who are obese/overweight also have poor metabolic control, as evidenced by a higher HbA1c (Maffeis et al, 2018; RCPCH, 2020).
Obesity in children with T1D is both influenced and complicated by the insulin administration and intensive dietary management that aim to maintain euglycaemia. Insulin is an anabolic hormone; in excess it can promote appetite and weight gain in addition to predisposing to hypoglycaemia. In contrast, the ingestion of sugary foods (or glucose) is mandatory for hypoglycaemia management. Thus, the longer the duration of diabetes, the greater the incidence of overweight and/or obesity (De Keukelaere et al, 2018), and this is more pronounced in females than males.
Management options
Medication
Metformin is licensed for children above 10 years of age. A few small studies have reported its use in children and adolescents with T1D and obesity, but the results have shown variable efficacy (Hamilton et al, 2003; Libman et al, 2015; Al Khalifa et al, 2017). The most recent systematic review on the usefulness of metformin for managing diabesity in children with T1D reported a clinically small, but statistically significant, reduction in obesity; a mean reduction of 1.46 kg in weight (p=0.008) and 0.1 reduction in BMI Z score (p=0.04) (Al Khalifa et al, 2017). It showed no change in mean HbA1c level (+0.05%; p=0.68) but a reduction in insulin requirement (–0.16 units/kg/day; p<0.0001) and a trend towards increased hypoglycaemic episodes (RR 4.21; p=0.06) (Al Khalifa et al, 2017).
There are no published studies on the use of orlistat, the only other medication licensed for managing obesity in children.
There is one small study on the use of dapagliflozin, a sodium-glucose co-transporter 2 inhibitor that increases glucosuria and thus improves hyperglycaemia in T2D patients (Roman et al, 2016a). In this study, three adolescent females with T1D used dapagliflozin and achieved an initial weight loss at 3 months, but partially regained the lost weight by 12 months. One patient stopped dapagliflozin due to a medication-related adverse event – euglycaemic diabetic ketoacidosis (Roman et al, 2016b).
Bariatric surgery
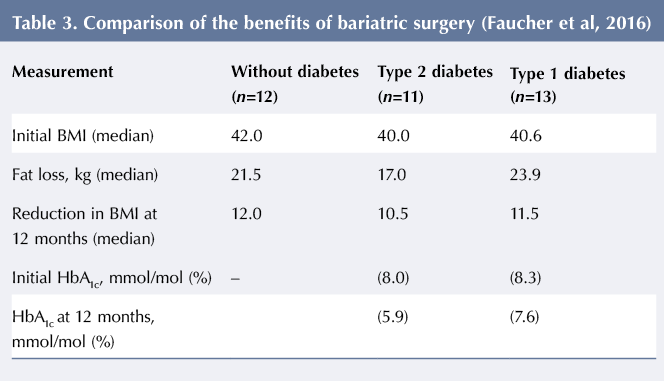
Bariatric surgery in young people with T1D is not recommended before 16 years of age, in view of a conservative and cautious approach. However, one small study comparing the benefits of bariatric surgery in obese adults with T1D, T2D and no diabetes showed comparable benefits in all three groups by the end of 1 year, see Table 3 (Faucher et al, 2016). The median HbA1c improved from 64 mmol/mol (8.0%) to 41 mmol/mol (5.9%) in T2D patients, with a beneficial reduction in medications from three to none in those who were on insulin and from 10 to four in those who were taking oral medications. Similarly, in T1D the median HbA1c improved from 67 mmol/mol (8.3%) to 60 mmol/mol (7.6%), with a reduction in mean insulin dose from 0.8 units/kg/day to 0.45 units/kg/day and with all five patients discontinuing metformin (Faucher et al, 2016).
Management challenges
There is very little published evidence about the optimum management of diabesity in T1D but there is strong evidence that increased physical activity is beneficial for blood glucose homeostasis and the prevention of obesity in T2D patients. In T1D diabesity there are no long-term studies on the benefits of healthy living and lifestyle interventions, such as low-calorie diets or exercise. One pragmatic approach is to achieve a balanced reduction in calorie intake, along with appropriate and balanced insulin reduction, in order to avoid hypoglycaemia. This is often best done with multidisciplinary team support with the dietitian taking a lead role in meal planning with balanced calorie and insulin reduction.
Adolescents with T1D, predominantly females, have a strong desire to maintain weight and avoid being overweight. In a survey of Swedish teenagers aged 15–18 years, the most important aspect for them was avoiding weight gain, although both hypoglycaemia and hyperglycaemia were reported as undesirable. Interestingly, female adolescents were willing to compromise their diabetes care and accept a mean HbA1c increment of 13.2 mmol/mol (1.2%) for a modest weight loss of 3 kg (Forsander et al, 2018).
Conclusions
Prevalence of diabesity in children with T1D is increasing and is associated with a greater risk of morbidity. This is a public health issue with potentially high societal cost. Research addressing the prevention of obesity and the optimal treatment and management of children and young people with T1D is warranted.
Acknowledgement
Sincere thanks to Mr Igor Brbre, clinical librarian, and Brighton and Sussex NHS Library and Knowledge Service, for facilitating systematic evidence search on type 1 diabetes in children and obesity.





NHSEI National Clinical Lead for Diabetes in Children and Young People, Fulya Mehta, outlines the areas of focus for improving paediatric diabetes care.
16 Nov 2022