The recent International Society for Paediatric and Adolescent Diabetes (ISPAD) conference saw the rise of automated delivery systems and technology in the management of type 1 diabetes (T1D) in children and young people (CYP). Sessions discussed how the systems work and considered how advice and education about glucose management may need to adapt to optimise their use.
Automated insulin delivery systems
Many studies have shown the benefits of using automated insulin delivery systems (hybrid closed-loop systems) in CYP with T1D (Weinzimer et al, 2008; Hovorka et al, 2010; Elleri et al, 2011; Bekiari et al, 2018). All have shown improvements in blood glucose time in range (TiR) and HbA1c but some have shown increases in hypoglycaemia. Users and families frequently report initial positive psychosocial outcomes. One small longer-term follow-up study, however, has suggested a decrease in system use over 12 months (median automode use decreases from around 80% to <2%) (Lal et al, 2019). It is thought that the need for calibration, the alarms and failure of the closed loop all lead to discontinuation.
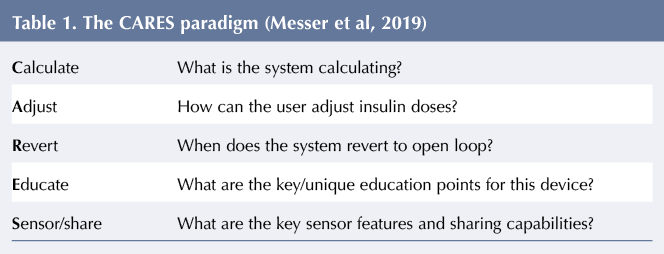
There are currently two US Food and Drug Administration-approved delivery systems for use in CYP: Medtronic 670 g and Tandem t:slim with Control-IQ (not yet available in the UK). Laurel Messer (Colorado) compared the two, highlighting the minor differences that, although small, have clinical implications relating to how the devices affect the management of blood glucose. It is essential that healthcare practitioners (HCPs) advising CYP with T1D understand the differences in order to optimise use of these systems. When initiating automated insulin delivery, Messer’s team uses the CARES paradigm, see Table 1, which prompts HCPs to consider the parameters of the system being used (Messer et al, 2019).
The key strategies that need to be implemented alongside any automated insulin delivery system are:
- The bolus should be based on an accurate insulin-to-carbohydrate ratio, consistent carbohydrate counting, and given at least 10–20 minutes pre-prandially. (Other speakers showed that it is consistency rather than accuracy in carbohydrate counting that optimises insulin bolus doses.) The bolus is most important for euglycaemia.
- All carbohydrates should be covered with bolus insulin. The algorithm will increase basal insulin and inappropriately adjust back-up basal patterns if this is not the case.
- Hypoglycaemia requires a smaller amount of fast-acting carbohydrate to return blood glucose to target range.
- Turning off the loop and reverting to backup basal pattern may be necessary when temporary basal rates are required (eg during sickness, when taking steroid medications or exercising) unless personal blood glucose targets can be adjusted.
- Alerts and alarms must be rationalised to avoid fatigue.
- Users should trust the system and avoid intervening.
HCPs need to be aware that automated insulin delivery systems may not be suitable for families or patients who like to finely manage their diabetes. Barriers to optimal system use should be anticipated and acknowledged on its initiation. Families will have expectations for outcomes, and HCPs will expect families to follow guidelines using new technology – these should be agreed at its onset.
How does food fit in with technology?
As technology evolves, the postprandial glycaemic effect of foods becomes more visible. Continuous glucose monitoring provides glycaemic information we would not see with discrete finger-prick data. This gives us the potential to adjust insulin delivery to optimise its effect. Symposia discussing continuous glucose monitoring data repeatedly called for the assessment of blood glucose TiR as a more useful marker than HbA1c of overall glycaemic control and risk of long-term complications. Postprandial blood glucose (PPBG) contributes to TiR and is a target for improved glycaemic control. It is important to recognise that a normal PPBG excursion can be within two standard deviations of baseline; when setting a target range, realistic blood glucose levels should be used to reflect this.
Food choices, meal planning and eating routine are known to influence glycaemic outcomes, but which factors most affect PPBG? Olga Kordonouri (Hannover) summarised the evidence:
- Preprandial insulin, ideally rapid-acting insulin, should be given 20 minutes before eating. This is safe in children of all ages. In toddlers, where there are often concerns over the unpredictability of eating, pre-bolusing rarely leads to hypoglycaemia. If it does, it is usually 2 hours later, which allows a window for preventative action when additional carbohydrates can be taken.
- Lower glycaemic index carbohydrates avoid spikes in PPBG levels, better matching the profile of rapid-acting insulin.
- Eating >75 g of protein alone (300 g steak) or 12.5 g of protein with carbohydrate will have an independent and additional glycaemic effect approximately 4 hours after eating. Fat enhances the response to protein. Giving extra insulin for fat and protein is increasingly being included in education programmes, especially for patients using insulin pumps. Current evidence allows us to offer a safe starting point for the calculation and optimal administration of fat–protein insulin that can then be adjusted to meet the patient’s PPBG response.
- Eating at regular times rather than grazing has a more positive effect on HbA1c than the macronutrient composition of a diet. This is likely due to improved TiR.
Exercise
Michael Riddell (Canada) gave a whistle-stop lecture about all things exercise and T1D. All CYP should be getting 60 minutes of physical activity per day, which should be at a moderate to vigorous level three times a week. (The majority of this population group in the UK are not close to achieving this target.) In CYP with T1D, this requires a balance between insulin and diet to keep blood glucose in target range during and after physical activity.
Outlining exercise physiology in trained and untrained athletes, we heard that individual variability is a real challenge in advising CYP with T1D on strategies to avoid hypo- or hyperglycaemia during exercise. We may attempt to predict the glycaemic effects of different types of exercise (aerobic causing hypoglycaemia and anaerobic causing hyperglycaemia) but individual responses will vary. However, in individuals responses do seem to be reproducible. HCPs may recommend trying a number of insulin adjustment or carbohydrate fuelling strategies for physical activity to identify which works best. The individual can repeat this strategy for reliable glycaemic outcomes when participating in exercise of the same type in future.
Hypoglycaemia is a commonly-reported fear around exercising with T1D and cannot be predicted by fitness status. Risk can be reduced by:
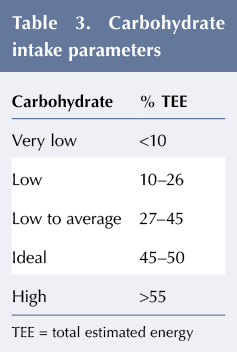
- Ensuring optimal glycogen stores, see Table 2. The effect of low-carbohydrate diets (LCDs) on glycogen stores is not really known and is currently under investigation.
- Reducing circulating insulin during exercise (making basal and bolus adjustments 90 minutes before exercise, where possible, and if not then providing pre-exercise carbohydrate).
- Using a 10-second maximal sprint during aerobic exercise to buffer blood glucose levels by switching to anaerobic respiration. However, a certain level of fitness is required to enable the athlete to achieve anaerobic respiration. This technique can be analogised to ‘spending on a credit card’, using up stores of liver glycogen that must be replaced soon after exercise to prevent delayed hypoglycaemia.
- At bedtime, circulating insulin can be reduced for 4–6 hours at the onset of sleep to avoid nocturnal hypoglycaemic events. Alternatively, ingesting whey protein (the optimal amount has yet to be decided) can delay nocturnal hypoglycaemia.
A summary of advice about exercise and T1D has been published by ISPAD (Adolfsson et al, 2018). This is essential reading for all HCPs working with CYP with diabetes, who should be encouraging more physical activity in their patients.
LCDs in CYP with T1D
LCDs were discussed in the final nutrition symposium by Belinda Lennerz (USA) and Carmel Smart (Australia). This topic is increasingly being raised in diabetes clinics internationally. Current ISPAD guidance recommends that CYP with T1D obtain 45–50% of their daily total estimated energy (TEE) from carbohydrates. However there is an emerging trend for families to follow a diet lower in carbohydrate for youngsters with T1D.
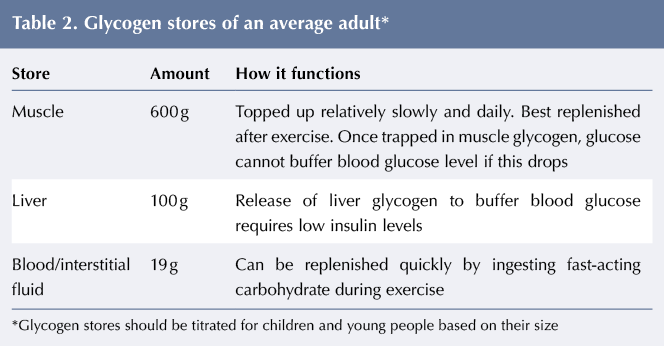
The definition of a LCD varies. Families who report they ‘low carb’ may not be restricting carbohydrate to a concerning level. Children’s requirements vary according to age, ie 50 g carbohydrate per day for a 5-year-old does not represent the same restriction as the same daily intake for a 16-year-old. Therefore defining a diet by carbohydrate content alone can be misleading. It is suggested that carbohydrate as a percentage of TEE is a better indicator of dietary adequacy, see Table 3.
LCDs and ketogenic diets should not be confused. Ketogenic diets replace carbohydrate with fat sources and intend to generate high levels of ketones. LCDs include more carbohydrate and protein, thus keeping ketosis at lower levels.
Lennerz summarised the findings of a questionnaire she circulated to members of an online T1D community following a LCD with T1D (Lennerz et al, 2018). The most common beliefs among respondents were that the LCD provided better glycaemic control and helped with weight management, but there is little evidence to support this in comparison to regular dietary advice and insulin dose adjustment for those with T1D. Smart went on to demonstrate that LCDs are not necessary for optimal glycaemic control and that the unique nutritional needs of CYP are difficult to achieve when carbohydrate intake is restricted.
Pros and cons of LCDs and very LCDs
Studies have suggested that glycaemic benefits may result from carbohydrate restriction in adults with T1D but in children there are no equivalent studies. Restricting carbohydrates in children increases the risk of macro- and micronutrient (energy, vitamin C, calcium, fibre) deficiency. This may lead to stunted growth and other long-term complications, such as low bone mineral density, as has been reported in children following therapeutic ketogenic diets.
When LCDs are followed, reduction in insulin doses results in lower levels of circulating insulin. Insulin has a role in the anabolic–catabolic cycle; its presence promotes anabolism. When insulin levels are low, cells switch to a catabolic state of starvation that generates ketones, blunting hypoglycaemia awareness and the response to glucagon rescue, increasing the risk of severe hypoglycaemia with LCDs (Ranjan et al, 2017). Habitual low levels of ketones on very LCDs means that diabetic ketoacidosis with euglycaemia is also a significant risk, especially at times of illness or decompensation.
A series of case reports of youngsters using LCDs highlights the significant psychological distress that can be caused by the endocrine and metabolic consequences of LCDs (De Bock et al, 2018). CYP with T1D are already very food aware – counting carbohydrates and maybe fat and protein at every meal. This increases the risk of disordered eating. Further restriction or strict control of their diet could increase this risk further.
Achieving blood glucose targets without LCDs
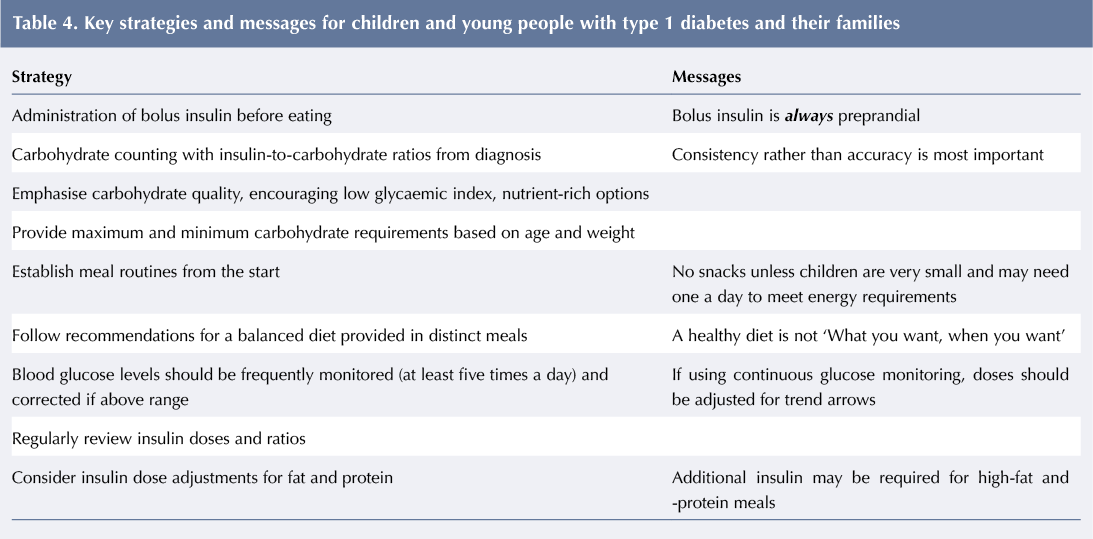
An audit of an Australian paediatric diabetes clinic showed how, through education about how to best match bolus insulin to food, on average CYP can get 48% TEE from carbohydrate and still achieve HbA1c of 46.5 mmol/mol (6.4%). Although eating habits and routines vary, generally insulin can be adjusted for them all. Key strategies and messages are given in Table 4.
As HCPs, we have a responsibility to explore the risks and benefits of dietary choices with families and CYP, especially those considering or following LCDs. This information is invaluable in allowing open and informed conversations with patients and families to guide them in their decisions. If they do choose to follow a restricted diet then we should to continue to support them but ensure that micronutrient levels and growth are monitored, dietary analysis is undertaken, and any deficiencies supplemented to meet nutritional requirements. They should also be given extra safety advice for times of illness when ketoacidosis can occur rapidly.
Summary
The evolution of technology in diabetes is changing the landscape for dietitians and other HCPs working with the increasing numbers of CYP using it. Technology requires our understanding to evolve and the provision of tailored advice to ensure it is optimally used. However, as we heard at ISPAD, despite new technology, the key dietary messages are the same as they have always been.
Exercise is often overlooked in diabetes management plans, but we now have a far better understanding of its effects on glycaemia in those with T1D, allowing us to create management plans that provide CYP with confidence that they can minimise exercise-related dysglycaemia. We should be enquiring about activity levels, encouraging CYP to meet the current recommendations and feel confident advising patients how to adjust their insulin and carbohydrate intake accordingly.
Dietitians will always be integral to teams providing diabetes care and education. While our dietary advice is still relevant, we need to adapt it with the times and use our role to encourage healthy living habits around diet and exercise that CYP can carry with them through life.




NHSEI National Clinical Lead for Diabetes in Children and Young People, Fulya Mehta, outlines the areas of focus for improving paediatric diabetes care.
16 Nov 2022