Q: Adoption of glucose-sensing technology, including flash monitoring, is increasing rapidly in the UK, although there is still some geographical variation. To what extent is this down to a lack of specialist skills to support it amongst healthcare professionals (HCPs)? How might this be improved?
A: The rapid increase in demand for glucose-sensing technology, prompted mainly by initiatives from NHS England to extend eligibility for these technologies (for example by mandating the use of CGM in pregnancy for women with type 1 diabetes and those with type 2 diabetes on intensive insulin regimens), has inevitably exposed the fact that there is variation in the capacity and expertise of services to deliver these technologies to their patients.
Whilst in the past access to insulin pump therapy was limited in some places by suspicion about the safety and reliability of the technology, this is not really the case for glucose monitoring technologies, so the lack of specialist skills is probably the most important limiting factor.
To address this deficit, the Diabetes Technology Network has created a suite of educational modules to support healthcare professionals in developing the necessary skills to support their patients using these technologies, and these can be accessed via the Glooko Academy platform.

Q: Healthcare systems and indeed guidelines are still focused on HbA1c. How might you translate Time in Range to HbA1c?
A: There is a good correlation between Time in Range (TIR) and HbA1c, which has allowed data from glucose-sensing technology to be converted into an estimated HbA1c. This estimated measurement may be particularly useful for patients in whom HbA1c testing is unreliable, such as those with haemoglobinopathies and chronic kidney disease.
Analyses of glucose sensor data suggest that a 70% TIR (3.9–10 mmol/L) equates approximately to an HbA1c of 53 mmol/mol (7.0%). Each 10% change in TIR equates approximately to an HbA1c change of 5 mmol/mol.
However, it is important to recognise that TIR is not simply a surrogate for HbA1c. It also provides valuable information about day-to-day glycaemic variability and is a much more accessible metric for the person using CGM, as they can get positive reinforcement as to the effectiveness of therapy changes much more quickly than from serial HbA1c measurements.
Q: CGM produces a huge amount of data, which could be overwhelming not only for the individual but also for the HCP. How do you unravel this and ensure you adopt a structured approach to the interpretation?
A: The uploaded data from CGM is displayed in several ways, so a structured approach to interpretation is needed.
The summary page shows the metrics, usually from a default period of two weeks (Figure 1). TIR can be reviewed immediately; if it is on target then there is little need to change anything unless the patient has specific concerns, such as severe hypoglycaemic episodes. These too can usually be quickly assessed, focusing on particular times of day when hypoglycaemia occurs or events which might result in hypoglycaemia.
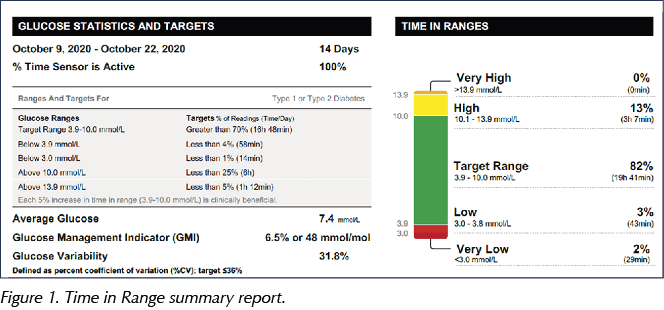
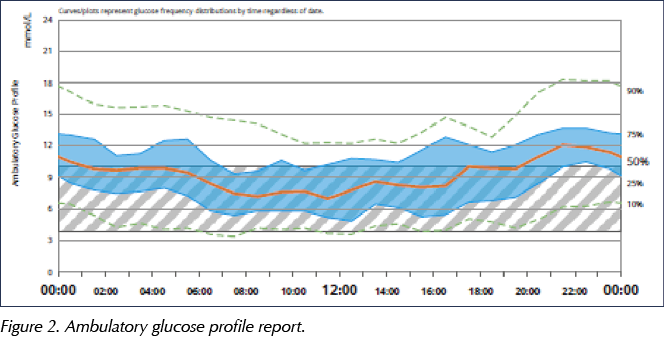
If the TIR metrics are not to target, the next thing to look at is the ambulatory glucose profile (AGP; Figure 2), trying to identify any consistent patterns which can inform treatment changes. However, it is important to recognise the limitations of the AGP and not to over-interpret this information.
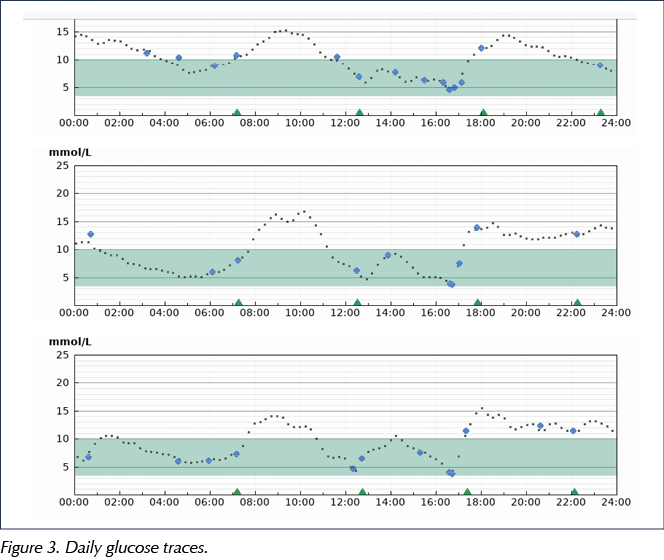
Finally, a review of the daily glucose traces (Figure 3), alongside information about insulin dosing, carbohydrate intake and other factors which may impact on glucose control, can be undertaken.
More details on this structured approach can be found in a review by Millson and Hammond (2020) and an accompanying quick guide in this Journal.
Q: It isn’t easy making sense of ambulatory glucose profiles – there seems a lot of detective work required. How labour-intensive is this? Is it something that all DSNs should be capable of doing?
A: I think all DSNs who are analysing glucose monitoring data should at least understand what the AGP is showing. It averages glucose data over a two-week period to show a median glucose level, with shaded areas on either side usually representing the 25–75th centiles and the 10–90th centiles (Figure 2).
Where the shaded areas are relatively tight around the median glucose and the centile lines follow a similar trajectory to the median, then the trend shown by this averaged data is likely to be a consistent pattern and so can be used to inform therapy changes. This is often the case with the overnight pattern, but the variability is usually much greater through the day and particularly during the evening, reflecting the fact that mealtimes may vary considerably from day to day; thus, the AGP is often of more limited value at these times.
My advice would be to have a sufficient understanding of the AGP to be able to assess how useful the report is with a quick glance and then to move on to other data if its value is likely to be limited.
Q: This is not something we typically do in primary care. But where do you see the role of primary care in terms of supporting these types of diabetes technologies?
A: I think primary care has a very important role in identifying people with diabetes who would benefit from the introduction of diabetes technology, be that insulin pump therapy, CGM or both. There was a trend 15–20 years ago for people with type 1 diabetes who were regarded as stable to be discharged to primary care. We now prefer all patients with type 1 diabetes to have some form of regular contact with the secondary care diabetes service, but there are still many people with type 1 diabetes who are only seen in primary care. These people will often not have any knowledge of how diabetes technology could help them. They may have good control but can only achieve this by testing capillary glucose levels eight or more times a day and/or at the expense of frequent hypoglycaemia, which they may be less aware of as their duration of diabetes increases. These individuals should definitely be offered the FreeStyle Libre and in due course may benefit from other technologies.
HbA1c, although the most reliable marker of long-term complication risk, is a blunt tool in identifying the challenges of living with diabetes on a daily basis, and recognising this will help in looking for other issues of diabetes management that might be helped by introducing technology.
Once people with type 1 diabetes are using diabetes technology, primary care can also play a role in supporting them to use the technology optimally. There may be infrequent follow-up in the secondary care service, so when the individual is seen in primary care issues may be flagged, such as how best to optimise meal-time insulin delivery or how to manage insulin dosing and carbohydrate intake with exercise. The Diabetes Technology Network modules are designed to be used by both healthcare professionals and technology users, so it will be useful for primary care professionals to have an understanding of what issues these modules cover, so that they can signpost users to those which might be of benefit to them.
As the criteria for accessing diabetes technology extend to include people with type 2 diabetes, as they inevitably will, primary care will have an even more important role, as these patients will often be seen exclusively in primary care, and so understanding which patient cohorts should be considered for glucose-sensing technology will be crucial to allow them to gain appropriate access.
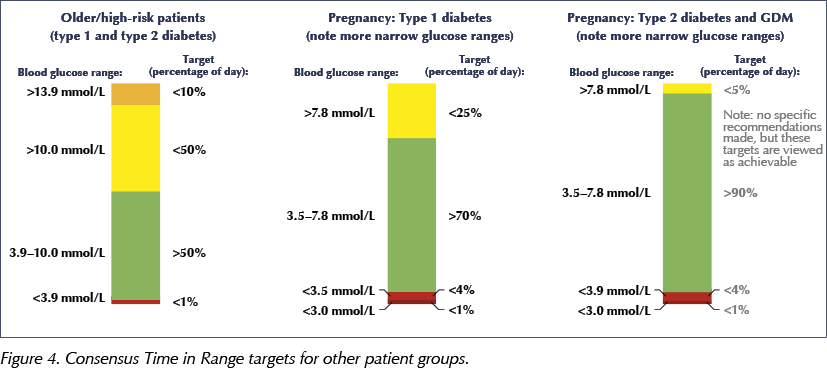
Q: The International Consensus on Time in Range (Battelino et al, 2019) has targets for various patient groups, including narrower glucose ranges for pregnancy, both in women with type 1 diabetes and in those with type 2 diabetes and gestational diabetes (Figure 4). In practice, is CGM, or indeed flash glucose monitoring, being offered to women with type 2 or gestational diabetes?
A: The funding ringfenced for CGM in pregnancy by NHS England is for women with type 1 diabetes. However, there is good evidence that women with type 2 diabetes on intensive insulin regimens will also benefit from the use of glucose-sensing technology, so this option should certainly be considered for these patients.
For most women who are managing their gestational diabetes with metformin alone or with basal insulin, target glucose control is achievable with capillary glucose monitoring on its own; however, if this is not the case and they are requiring multiple insulin injections to manage the condition, a glucose-sensing option should be considered.
In summary, all women with type 1 diabetes should be using CGM during pregnancy. For pregnant women with type 2 or gestational diabetes, the need for a glucose-sensing solution should be made on an individual basis and will be limited to those on an intensive insulin regimen.
Q: If women with type 1 diabetes are encouraged to use CGM in pregnancy but then that technology is taken away (as per guidelines), isn’t that quite demotivating and are there ways of justifying ongoing use?
A: This is certainly going to be a challenging problem for services: taking away a potentially helpful technology is not going to be easy. There is definitely justification for continuing to make CGM available whilst women are vulnerable to hypoglycaemia, for example whilst breastfeeding. Furthermore, where CGM can be shown to have provided benefit over and above the FreeStyle Libre in protecting against severe hypoglycaemia then it would be reasonable to continue CGM after pregnancy.
In addition, the fact that many of these women will be eligible to use flash glucose monitoring after they come off CGM, and that flash monitoring is evolving – with the Libre 2 having alarms and the next version, Libre 3, being a CGM device – means that coming off CGM will be less of an issue than it would be if the user had to revert to capillary glucose monitoring.
Q: What are the current legal requirements with respect to CGM/flash glucose monitoring and driving?
A: The DVLA announced in February 2019 that it would recognise CGM and flash as acceptable methods of glucose monitoring whilst driving for car and motorcycle (Group 1) drivers. However, for lorry and bus (Group 2) drivers, anyone who is using insulin or other agents that carry a risk of hypoglycaemia must continue to use capillary glucose monitoring at regular intervals whilst driving, and at least twice daily even on non-driving days (see March 2021 update of Assessing Fitness to Drive guidance).
Where a driver is using glucose-sensing technology, they must check their capillary glucose if their sensor glucose level is 4.0 mmol/L or below, if they experience symptoms of hypoglycaemia or if the sensor gives a reading that is not consistent with the symptoms they are experiencing.
Q: As life expectancy increases, we are seeing older people with type 1 diabetes – individuals in their 80s and 90s. Does this technology have a place in these age groups?
A: There are plenty of people in these age groups who do use these technologies, and glucose-sensing technology in particular can have a valuable role even in those with cognitive impairment. We have successfully used CGM in hypoglycaemia-prone elderly people with type 1 diabetes and cognitive impairment, linking close friends and family to the data so that they can potentially take action when it appears that the person themselves has not responded appropriately to falling glucose levels. We also see an increasing number of people in this demographic who are tech-savvy and in good shape physically and mentally, and they welcome the opportunity to start or continue using glucose-sensing technology.
It is much less common to start insulin pump therapy in this age group, although this should not be dismissed as an option just because of someone’s age. Indeed, the advent of hybrid closed-loop systems could change our approach in this age group, as these systems could considerably reduce the impact and risk of diabetes in the elderly. However, we also have to be realistic in this age group, and where continued use of diabetes technology, particularly insulin pump therapy, presents an increasing cognitive challenge, a sensitively handled transfer back to insulin injections is the best course of action.
Q: One of the things I’ve learnt over the pandemic is that digital technology is not everyone’s cup of tea! Have you found that some people simply find the technology too intrusive in terms of being constantly “connected” and also being forced to share data? What would you recommend for this group?
A: You should never force technology on someone who does not want to use it. Living with type 1 diabetes is a huge challenge and the person with type 1 diabetes has to use the management tools that best suit them and their lifestyle.
If the issue is simply a concern about sharing data then a compromise can often be reached, whereby the data is only shared at a clinic appointment, where it is looked at together.
If you think someone would really benefit from a particular technology but they have significant reservations, it can be really helpful to direct them to peer support – talking to someone who has themselves used and benefited from the technology may be the eye opener needed to change their preconceptions.
Q: What support is out there for people using insulin pump therapy?
A: The Diabetes Technology Network education modules cover insulin pump therapy, and there is some really helpful information for someone who is considering using this technology, both in explaining what pump therapy is all about and in helping them to choose the pump that is right for them.
Q: If we future-gaze for a moment, do you think we’ll look back and see finger-prick testing as archaic, in the same way we now view urine testing for measuring glucose? Do you think flash monitoring, in particular, will become the norm for people with type 2 diabetes too?
A: I think it is inevitable that, as more manufacturers enter the continuous glucose-sensing market and competition drives down the cost, CGM/flash will become the norm for everyone with type 1 diabetes and those with type 2 diabetes for whom glucose monitoring is indicated. The only constraint at present is cost – if there were no excess costs with sensor technology, we would not think twice about using it whenever glucose monitoring was needed.
There is a huge difference between a one-off capillary glucose reading, providing no information about the direction of change in glucose levels, versus the information from a glucose sensor reading, which tells the user not only their present glucose level but also the rate and direction of change, and even predicting when they might expect to become hypoglycaemic. This does not even take into account the wealth of information that sensor data provides when looked at retrospectively to help optimise glycaemic control. We already see that using flash glucose monitoring often results in a significant improvement in HbA1c (Deshmukh et al, 2020), and if ongoing audit data confirm that this effect is sustained then we may well see a cost-effectiveness case approved for flash to be made available to everyone with type 1 diabetes, even without a reduction in the current cost.





Helping homeless adults to overcome the challenges of managing their condition.
16 Apr 2024