Diabetes is considered the most prevalent disease within the UK. The number of people in England, diagnosed with diabetes rose by 53% between 2006 and 2013, and the National Institute for Health and Care Excellence (NICE) estimates that 5 million people will be diagnosed by 2025 (NICE, 2015). Ten per cent of people with diabetes will develop a foot ulceration and 80% of this population will require non-traumatic limb amputation because of osteomyelitis (OM) (NICE, 2015), costing the healthcare service anywhere between £3,000 and £65,000 per ulceration (Strategic AHP Lead Group, 2014).
Accurate and timely diagnosis of osteomyelitis within a diabetic foot ulcer (DFU) is paramount in any healthcare setting due to the dire consequences that can occur if it is left undetected and untreated. Diagnosis begins in a clinical setting, with recognition of a wound that probes to the bone and the presence of two or more of the six signs of infection: pain, erythema, heat, oedema, exudate and malodour. These signs arouse suspicion and the call for a constellation of further investigations. Imaging modalities, such as X-rays, are one of them and are regularly used to help confirm, refute and map the diagnosis, progression and treatment of OM (Aragón-Sánchez et al, 2011).
Diagnosis of diabetic foot infection
The ability to diagnose diabetic foot infection (DFI) is a challenging, but essential, skill for any clinician. Curbing a DFI in its infancy promotes a greater chance of the ulceration healing and not progressing and developing sequelae such as OM. A diagnosis of infection is confirmed by a collection of clinical signs and symptoms, and should not be made on just microbiological results alone; Richard et al (2011) emphasise that all open wounds will be colonised to some degree, but not all will be infected.
Clinicians are constantly inspecting DFUs for the six signs of soft tissue infection and Lipksy identified that the longer the infection is present, the more widespread and intense the clinical signs will be and the greater the severity (Lipsky, 1997). In 2012, The Infectious Diseases Society of America released validated clinical criteria for the recognition and classification of DFIs (Table 1), which have now been recognised as best practice by Wounds International, the NHS and NICE.
DFIs are notorious for containing a complex polymicrobial flora, such as as aerobic Gram-positive cocci, Gram-negative bacilli (e.g. Escherichia coli, Klebsiella species and Proteus species), and anaerobes (e.g. Bacteroides species and Peptostreptococci) (Hunt, 1992). With the skin closed, these microorganisms are harmless. However, people with diabetes suffering from macro- and microvascular comorbidities, neuropathy and friable skin overlying prominent bony regions of the foot are highly likely to develop ulceration, allowing these microorganisms access to the subcutaneous structures, such as bone (El-Tahawy, 2000).
Diabetic foot infections and osteomyelitis
Early OM is rather difficult to diagnose. Table 1 indicates that OM is suspected if a DFI is graded as 3 or 4. The wounds will be chronic, large and deep in nature, present with purulent discharge, expose bone or presents like a sausage toe (Wounds International, 2013).
OM is classified as either acute or chronic and is characterised by progressive inflammatory destruction of bone (necrosis), followed by apposition of new bone in a bid to confine the inflammation (Marais et al, 2013). Puzas et al (1994) highlighted that bacterial components and toxins have an influential stimulatory effect on osteoclastic activity which, in turn, inhibits the action of osteoblasts, consequently evoking bone reabsorption at the site of infection (Puzas et al, 1994). This reabsorption of bone can lead to pathological fractures and deformity within the foot.
Assessing the involvement of bone begins clinically with a wound that has a positive probe to bone (PTB) test. In 1995, Grayson et al described a relatively easy and highly sensitive technique whereby of a blunt metal probe was used to explore the cavity of a DFU and indentify any presence of palpable bone. They coined the phrase “probe to bone test”. Unfortunately, this developed a ‘hammer and nail’ approach to the diagnosis of OM in the diabetic foot and resulted in large amounts of inappropriate antibiotic use (Lavery et al, 2007).
Subsequently, Lavery et al (2007) suggested that the PTB is a better tool to exclude the presence of OM from a DFU than prove it. Therefore, if bone is palpated in a typical clinical setting this should arouse suspicion of OM, not encourage confirmation. The suspicion created by a positive PTB evokes a cascade of different clinical diagnostic investigations to help confirm the presence of OM. These include:
- Bloods (full blood count, erythrocyte sedimentation rate, C-reactive protein, HbA1c, liver function tests and urea and electrolytes) to identify any elevation in serum inflammatory markers
- White blood cell counts
- Increase in blood glucose levels and changes to organ function, such as the liver and kidneys
- Deep wound swabs
- Tissue samples and diagnostic imagery.
One area of clinical investigation that can be regularly overlooked is the patients themselves. OM is a precursor to sepsis and in a critically ill patient, temperature changes ±1°C can be enough to establish sepsis and the need for time-sensitive emergency care (Bota et al, 2004; Drewry et al, 2013). Therefore, regular use of the early warning chart on all patients with a DFU is encouraged. The Royal College of Physicians strongly advocates the regular use of this chart so as to track a patient’s clinical condition and trigger a timely medical response before deterioration (NHS Diabetes, 2010). This score measures a patients respiratory rate, oxygen saturations, temperature changes, blood pressure, pulse rate and level of consciousness. A figure is allocated for each, and the magnitude of that figure signifies the severity of each measure (Royal College of Physicians, 2012).
One further factor that needs to be taken into account is wound location. Wounds that are found on the digits have very little soft tissue underlying the skin; therefore, the potential for exposed bone is greater in comparison to that of a wound on the plantar surface near the heel. A clinician who undertakes the PTB will automatically take into account the texture and density of the bone. Bone that is hard and smooth can be seen as normal and infection-free, whereas a bone that has a rough, brittle density can be indicative of bone destruction. A diagnosis of OM occurs not just by clinical tests, but through clinical experience too (Lavery et al, 2007).
X-rays and osteomyelitis
When the clinical signs of infection are evident and the clinician suspects the presence of OM, the first step is to obtain imagery of the foot. NICE guideline 19 strongly recommends that an X-ray is obtained at the patient’s initial assessment to help confirm the presence of OM, but furthermore to give baseline images for comparison once treatment has begun (NICE, 2015).
X-rays are considered firstline because they are easy to access, quick, less invasive, can be repeated and cost relevantly little compared to magnetic resonance images (MRIs) and computed tomography scans. For a positive diagnosis of OM via X-ray, the infection needs to have been present for 10–20 days and bone absorption to be at least 40–70% (Lipsky, 1997). Prior to this, X-rays are not sensitive enough to confirm the presence of OM. MRI would be the most sensitive and accurate, but due to clinical time constraints, waiting lists and cost this is not achievable for all patients (Aragón-Sánchez et al, 2011; Richard et al, 2011). Importantly, clinicians should not solely rely on the radiological findings. This imagery is used to support the diagnosis of OM and clinical findings should not be ignored if a radiological report refutes the presence.
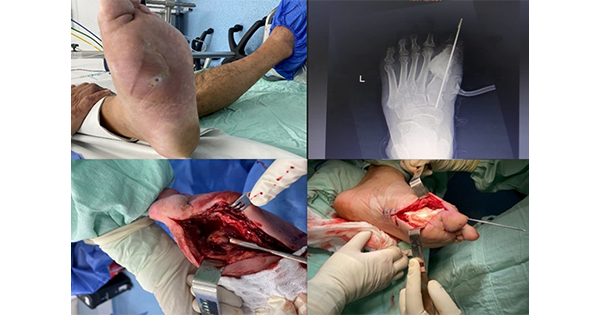
The radiological findings of acute OM are that of periosteal reaction, architectural changes to the trabecular pattern, regional osteopenia and focal lysis in the form of loss of cortical bone and cyst formation. Figures 1 to 3 help depict these changes. Chronic OM consists of sequestration of necrotic bone, involucrum and cloaca formation (Lipsky, 1997; Aragón-Sánchez et al, 2011; Nawaz et al, 2012). Although the latter radiological findings are not considered pathognomonic (present beyond any doubt), there is a rationale to initiate treatment for the presumption of OM, pending confirmation from a bone/tissue sample (Lipsky et al, 2012; Wounds International, 2013; NICE, 2015).
The use of serial X-rays is required to establish whether treatment is effective and map the progression of any further bone loss. NG19 offers guidance regarding the need for X-rays for DFI, but does not specify when and how often (NICE, 2015). NICE only suggests serial X-rays for the treatment of Charcot foot, but again offers no frequency. The Infectious Diseases Society of America identifies the need for serial imaging and recommends that if there are no radiological findings and the DFU is not healing it is necessary to repeat the X-ray every 2–4 weeks (Lipsky et al, 2012). This is not a hard and fast rule. Clinical experience will influence when to request a follow-up X-ray, and anecdotally this would be when a wound has deteriorated rapidly or the clinical infection fails to improve and the initial image is equivocal. If comparison of bone integrity is required to determine whether treatment (e.g. antibiotics) has been completed successfully then radiographs should be repeated with the same views at the same exposure level. A ‘like-for-like’ film is essential as harsh and dark X-rays can be erroneously interpreted as signs of pathology that are not present.
Capturing all three views (lateral, antero-posterior and oblique) of the foot at every radiological request is essential to present the foot’s true appearance. There are also a multitude of specialised views available. Figure 4 shows a plantar axial view, which successfully captures any sesamoid involvement with ulceration under the first metatarsophalangeal joint.
If the standard three views do not capture the anatomical location required, it is important to liaise directly with radiology at the request stage and ask for direction. This saves time and effort, and reduces repeat exposure of the patient to harmful X-rays. Additionally, the foot is a functional device and weight-bearing views are vital due to displacement of the soft tissues and the true position of the underlying bony structures when standing (Christman, 2015). This is evident in Figures 5 and 6. These images are of the same non-active Charcot foot, but yet Figure 5 is non-weight-bearing oblique, whereas Figure 6 is the weight-bearing lateral. This non-active Charcot foot has had chronic plantar ulceration for nearly a decade. Note the midfoot sag around the first metatarsophalangeal joint and the near normal alignment that the non-weight-bearing oblique view suggests; compare that to a weight-bearing lateral view with radiopaque marker in situ. The new view illustrates the true functional position of the bones and promotes the true Charcot deformity and cause of the chronic wound.
The use of a cutaneous radiopaque material to identify the wound’s location is strongly advised, but not regularly documented or used (DeSena, 1993). In practice, this takes minutes to apply, and marks the borders of the wound, highlights the area in question, and allows better scrutiny of the bony structures underneath by the reporting clinician.
Unlike the clinical practitioner, the reporting radiologist only has the images to report from, and the information given on the request form. The more relevant the clinical detail given, the more detailed and accurate a report will be produced. Finally, it is important to, discuss the report with the named reporter if something has been overlooked and clinical suspicion is still present. As any subtle appearance of the acute signs of OM (periosteal reaction, architectural changes to the trabecular pattern, regional osteopenia and focal lysis) should not be dismissed, even if not seen by radiology.
Conclusion
X-rays are a common tool used in the diagnosis and treatment of osteomyelitis. There are no set rules as to when a clinician should request them. Although X-rays are not seen as being sensitive in the acute stages, they provide the requesting clinician with a plethora of internal detail that cannot be seen clinically. Giving the radiology team a detailed request will allow better understanding of the image generated and a more accurate report. The use of all three views anterior-posterior/oblique and lateral in a weight-bearing stance, with wound markers, is advocated along with repeat X-rays when the ulcer changes or becomes static. Clinicians are reminded that although this article advocates the use of X-rays, other more sensitive imagery, such as MRI, should be ignored. OM is a challenging pathology to diagnose accurately and quickly. It is this authors’ recommendation that as a clinical team it would be best to produce a clinical pathway to manage such a complication so that all clinicians who visit can feel comfortable recognising, diagnosing and treating this complex sequelae of DFI.