The lack of a robust evidence base for both prevention and management has been well reviewed (Mason et al, 1999; De and Scarpello, 1999; O’Meara et al, 2000; Bradley et al, 1999; Valk et al, 2001; Jeffcoate and Harding, 2003). The reasons are clear. The diabetic foot is neglected by the majority of healthcare professionals, and particularly those who are more academically orientated. Diabetes is unfashionable and foot ulcers especially so. Ulcers are also managed by a wide range of carers (often several at the same time) including GPs, district nurses, diabetologists, vascular surgeons, community podiatrists, geriatricians and tissue viability nurses. Each has an incomplete perception of the totality of the problem, with opinions and management policies coloured by financial constraint and professional territorialism. Communication is generally poor, integrated care plans do not exist and there is little continuity of care. It is not a climate which fosters collaborative research.
The lack of interest by clinicians is mirrored by industry. Commercial interest is inevitably driven by potential profit, and profit is less predictable in a field with a poor profile among policy makers and clinicians alike. Moreover, the bulk of the revenue comes from dressing products, and the promotion of dressings (classified technically as ‘appliances’), which does not have to be substantiated by evidence of efficacy. There is less requirement to prove that they work than there is to convince people that they do.
However, clinical research is difficult without commercial backing, and it will be even more difficult when the guidelines for good practice in clinical research become legally enforced (in Europe) with the imminent adoption of the EU Working Directive 2001/20/EC. A team of administrative and secretarial staff is essential. Foot ulcer research will not be impossible, but it will pose a considerable intellectual and administrative challenge.
Difficulties inherent in planning research
Research into prevention is difficult because the endpoints are indirect, and the numbers needed to demonstrate an effect can reach into the thousands. For instance, a study of the benefit of education in primary prevention of ulcers in established neuropathy will need to be done in a total population of 1.5 million (42000 with diabetes, 14000 with neuropathy). Until such a study is done, we may believe that education prevents ulcers, but we will not know.
Research into management is made difficult by the complexity of the subject, with the multiplicity of factors contributing to the pathogenesis ensuring that the effect on outcome of any one intervention will be limited. This article reviews some of the issues and considers how the choice of outcome measure requires careful thought.
Study design
Studies are either observational or interventional. Interventional studies can be designed to determine efficacy, efficiency, effectiveness and/or cost-effectiveness (Panel 1). These distinctions are important as they reflect a spectrum between research which is more, or less, rigorously controlled on the one hand, but which is also less, or more, relevant to clinical practice on the other. For example, studies of the efficacy of a new product applied to the wound surface to promote healing, may be designed to eliminate all possible confounding influences on the outcome. It will be undertaken on ulcers of a particular type (e.g. uninfected plantar neuropathic ulcers) and be based on the use of very specific measures of response, such as the expression of enzymes and cytokines in punch biopsies taken from the wound edge. These may demonstrate an effect which is academically interesting, but which is not necessarily of clinical significance. Studies designed to establish clinical significance (effectiveness) have to be done in bigger, less selected, populations; these are harder to plan, and much more expensive to do.
Population selection
It is not possible to interpret the results of research unless the population, whether of people or ulcers, is clearly defined.
People
Populations vary enormously and this has a major impact on the outcome of research. For example, interpretation of the many epidemiological studies of the incidence of amputation in different communities is made difficult because of the influence of racial, social and economic factors, diabetes type and quality of control, and the structure of healthcare services. It follows that any such observational study must include precise details of the population studied. This is especially true when the observations relate to practice in specialist units, rather than whole communities. The population studied in specialist units will inevitably be selected, and the results can not therefore be extrapolated unless the basis of the selection process is known.
Ulcers
Populations must also be defined in interventional research. In this case, the term ‘population’ applies to both people and to their ulcers. Ulcers are of many different types (Figure 1), and subgroups need to be selected which share certain common properties. It is only when roughly similar ulcers are grouped that the effect of different treatments can be compared. The problem, as has been extensively debated in the past (Jeffcoate et al, 1993) is that there has been no classification in routine use which is robust enough for the purpose. The Meggitt-Wagner classification is vague and outdated. The Texas (San Antonio) classification (Armstrong et al, 1998) contains no reference to either area or neuropathy.
The S(AD) SAD classification system (Size [Area and Depth], Sepsis, Arteriopathy and Denervation) and the new PEDIS classification system (Perfusion, Extent, Depth, Infection, Sensation) are based on the subcategorisation of five key features of all feet with ulcers: area, depth, infection, ischaemia and neuropathy.
The S(AD) SAD classification (Macfarlane and Jeffcoate, 1999; Treece et al, 2004) includes both area and neuropathy, but uses relatively imprecise clinical criteria for grading and may be more useful for audit of large numbers than for research into management of well characterised groups. However, the new PEDIS classification, which has been designed purely for the purpose of research, offers promise (Schaper, 2004). PEDIS is the product of extensive international debate and while it is still in the evaluation phase, it could overcome many of the limitations of earlier systems.
Outcome measures
Outcomes can be divided into those that relate to cell biology, the ulcer, the limb, the person and the service, as can be seen in Panel 2 (Jeffcoate et al, 2004). In general, studies of efficacy use more targeted outcome measures (such as those of cell biology or ulcer-related) while studies of effectiveness should incorporate outcome measures which are relevant to the person, as much as to their foot.
(a) Cell biology
Measures of cell function will become progressively sophisticated and will stimulate increased knowledge about the wound healing process. However, there may be little immediate relevance to clinical practice.
(b) Ulcer
Both healing and non-healing of ulcers should be considered as outcome measures.
(i) Healing
While the relevance of healing is obvious, the term needs to be defined. Most would accept ‘complete epithelialisation without discharge’, but it is sometimes hard to be sure if this has occurred. As early breakdown is commonplace, studies ideally define healing as epithelialisation which is maintained for a fixed period, such as 28 days. Our experience is that 35% of all healed ulcers break down within a month, and these are obviously not regarded by either ourselves or our patients as having ever been properly healed. If not regarded as healed in practice, they should not be regarded as healed in research.
(ii) Non-healing
The term ‘non-healing’ is preferable to ‘failure to heal’. ‘Failure’ implies poor performance by either the cells of the wound bed or by clinicians, when both may have been doing their utmost in adverse circumstances. Moreover, the wound which is non-healed at the end of a fixed-term study may still be ‘healing’, albeit slowly. Non-healing is just as important an endpoint in clinical research as healing, and its proper analysis should encompass a number of possible options (Panel 3).
One group has recently used an immense database to emphasise the linearity of rate of wound closure in ulcers which go on to heal, and has suggested that rate of healing (decrease in ulcer area) can be used as a surrogate endpoint, enabling controlled trials to be shorter. Healing can be predicted rather than observed (Margolis et al, 2003). Inevitably, such a measure will be reserved for studies of the efficacy of an intervention.
(c) Leg
The principal leg-related outcome is limb salvage – either intact or following minor amputation. Amputation (whether major or minor) should never be taken as an endpoint in isolation. Some amputation wounds heal, and others do not, and ‘nibbling’ surgery is a well-recognised phenomenon. It is also acknowledged that survival after major amputation is not good (Faglia et al, 2001), and that some patients never leave hospital after the operation. Outcomes, whether the limb is removed or not, should be qualified by measures of function, (i.e. the usefulness of the leg/foot) and associated incapacity.
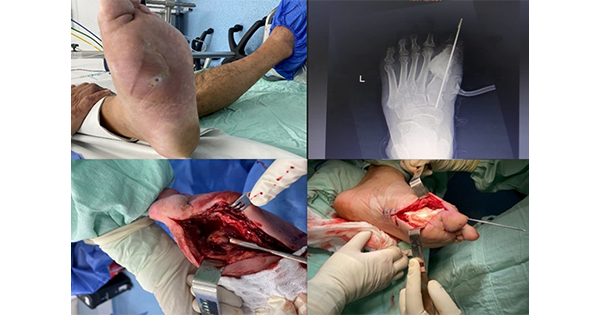
Amputation also causes deformity, and this deformity can itself lead to secondary ulceration (Figure 2). Murdoch et al (1997) reviewed the outcome following amputation of the hallux in 90 people with diabetes. They found that a second operation was required in 60% of participants within a mean of 10 months, and 17% eventually lost their leg. Another group reviewed the long-term outcome of amputation of one or more digits in 97 cases of forefoot infection. Recurrence was common and cure was achieved in only 34% (Nehler et al, 1999). Assessment of the effectiveness of amputation must therefore consider both short-term and long-term end-points, and measures of patient wellbeing.
(d) Person
Issues which may be considered include:
- Survival; the time of death and its relation to either the ulcer or its treatment.
- Persistent, recurrent or new ulceration. The ulcer assessed in any study of efficacy may not be the only one present. It may be of little consequence to the patient or the carers if the index ulcer heals while others persist. Similarly, the index ulcer may recur, often within the first 4 weeks, as described above. New ulcers may occur at other sites as well. Each ulcer presents a threat to the limb and to patient survival and while any persist, it matters little whether earlier ulcers have been successfully treated.
- Capacity/incapacity. There are no disease-specific measures of function (how much the person can do), although those available for stroke patients could be adapted, and there are generic scales (such as activities of daily living) which are in widespread use. The simple Euroqol-5D scoring system is relatively imprecise but is suitable for the study of large populations.
- Wellbeing. Disease-specific measures have been described and should become integrated into future prospective research (Price and Harding, 1997; Abetz et al, 2002; Vileikyte et al, 2003). Generic measures may also be used, including the Rand Short Form (SF12 or 36) scale or Hospital Anxiety and Depression (HAD) scale.
- Satisfaction. Measures of satisfaction tend to be of little practical value, because the baseline level of satisfaction with care is generally so high that it requires a very large effect for the difference to be detectable.
- Costs. Any health-economic evaluation must incorporate costs incurred by the patient and their carer.
(e) Service
While not directly relevant to the assessment of clinical effectiveness, some measure of cost and savings is an integral part of service planning and will inevitably be the focus for some future work.
Ulcer-free survival with limbs intact
In an attempt to encompass many of these issues, we have been exploring the concept of duration of survival, ulcer-free and with limbs intact. This is relatively simple to define, and as it is applicable even in routine practice, this measure goes some way to providing a marker of the overall effectiveness of care. At City Hospital, Nottingham, we have piloted its use by examining the outcome of 1013 patients on our database whose ulcers (total 3632) all healed at some stage. Recurrence or new ulceration was observed in almost 50% of patients, and nearly always within the first 12 months. However, the same data can be used to demonstrate that of those whose ulcers healed, 60% survived and remained ulcer-free and with limbs intact for a year, and almost 50% remained so for at least 3 years (unpublished data). The use of some such measure could be the basis for a comparison between units, provided that criteria for population selection were agreed.
The EU Directive
The EU Directive will make the requirements of research governance legally enforceable. Each study has a ‘sponsor’ (not necessarily the same as the funding body) who is legally liable for the work being properly done. The sponsor will therefore be instituting checks to ensure that every aspect of the conduct of a trial is checked and recorded according to recommended procedure. Each researcher will be inspected for each study on a regular basis, and will have to produce annual and final reports for the sponsor, the department of research and development and the funding body. Each study must have:
- A trial steering committee (largely independent) to which the trialist reports on an annual basis.
- A data monitoring and ethics committee (wholly independent) to which the trialist reports on an annual basis. (Anonymous, 2003)
Is there a future for clinical research in the field of the diabetic foot?
The answer must be: ‘yes, but it will be difficult’. If it had been easy, the necessary work would have been undertaken long ago. It will become even harder (in Europe, at least) when the EU Directive becomes law. Commercial, product-related research will continue, but the heavy administrative load means that non-commercial clinical research may only be possible if units link to form consortia with the required administrative staff, or if they use the services of a clinical trials unit, such as one of those established at a number of universities. Our aim is to establish a trial clinic which is designed for clinical staff who are interested in the field of the diabetic foot. The intention is that the ready availability of such a resource may help interested professionals overcome barriers which may otherwise appear insurmountable.
For more information about the Nottingham Foot Ulcer Trials unit visit: www.futu.co.uk