A consortium of interested clinicians has recently reported the results of the Charcot Disease in UK (CDUK) study in Diabetologia (Game et al, 2012), and this provides interesting new evidence concerning the causes and treatment of the acute Charcot foot. These results were derived from a web-based survey undertaken throughout the UK (and one centre in Ireland) that were recruited by advertisement in professional notices and publications, including those published in The Diabetic Foot Journal. Participating clinicians were then invited to register clinical details of all newly presenting cases of acute Charcot’s disease. The use of this approach resulted in data being available from 288 cases managed in 76 centres.
Causes
It is believed that acute Charcot’s disease is the result of an uncontrolled cycle involving inflammation, thinning of bones, fracture/dislocation and further inflammation (Jeffcoate et al, 2005; Rogers et al, 2011). While various factors may predispose an individual to develop Charcot, it is thought that the essential factor is distal neuropathy. However, there must then be a trigger to initiate the inflammatory cycle, and it has long been known that cases may be precipitated by ulceration or surgery. This was confirmed by the CDUK study, with 36% of cases recalling having experienced an episode of trauma in the 6 months before presentation (12% having had local surgery). Thirty-five percent had active ulceration at presentation, 20% of whom had concomitant osteomyelitis.
Treatment
There are two main treatment options available for Charcot: (i) off-loading with (ideally) non-removable devices, and (ii) the use of bisphosphonates. The CDUK study provided valuable, interesting information concerning both.
Off-loading
The use of non-removable off-loading devices is recognised as the treatment of choice for acute Charcot (Rogers et al, 2011) and it is therefore surprising that this method was used as part of the management in only 35% of cases registered in the CDUK study. The reason for this is obvious: the acute Charcot foot is managed by healthcare professionals who do not have access to the treatment that is recommended –
a situation that should simply not be tolerated.
Given this finding, questions must be asked about, and careful thought has to be given to, the organisation of future specialist care for the Charcot foot. If the opinion of patients and their representatives were sought, the answer would be clear: that they should either have access to the best available treatment in the place where they are managed, or they should be referred to another centre where such treatment is available. Referral to other centres will not always be what patients want, but it could certainly be argued that they should have the option.
Information was also sought on clinical outcomes, and the principal outcome measure used was the time to ambulation in usual or orthotic footwear. Outcome data were available in 219 (76%) cases in the series. The median time to normal walking was 9 months among those who were provided with some form of non-removable off-loading device at some stage during their management, and was significantly shorter when compared with the 12 months median time to normal working in the remainder (P=0.001).
Bisphosphonates
The evidence to justify the use of bisphosphonates has always been weak – even though it has been better than that available for much of the management of diabetic foot disease. Essentially, data from two small randomised trials (Jude et al, 2001; Pitocco et al, 2005) show that the use of either oral or intravenous bisphosphonate preparations is associated with a decrease in serum markers for bone turnover – which is a predictable consequence of their use.
Bisphosphonates were administered intravenously to 25% of the patients in the CDUK study, and orally in 19% (with some crossover between the intravenous and oral therapy groups). When the primary clinical outcome (time to ambulation) was compared between those who had, and those who had not, received bisphosphonates at some stage, it was found to be significantly shorter in those who had not been exposed to the drug (10 months vs 12 months; P=0.005).
Limitations of the CDUK study
The problem encountered in attempts to study either the cause or the outcomes of Charcot’s disease is its rarity. Even the biggest centres will see no more than 10–15 cases each year. The CDUK study attempted to overcome this barrier by the use of the Internet to undertake an audit of cases in as large a number of centres as possible.
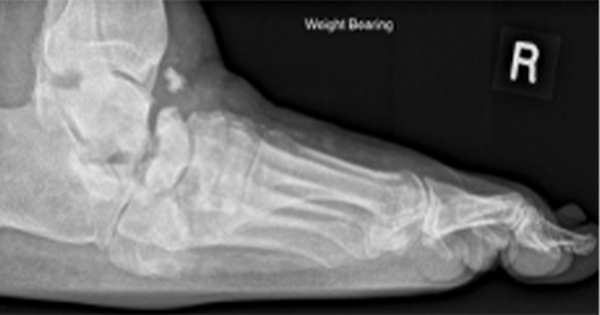
This approach has a number of limitations, which are primarily the result striking a balance between requesting sufficient information to be of value and the need for brevity – because contributors will be easily put off if asked to record excessive amounts of data, or for case details that are difficult to obtain. It was for this reason that this survey requested minimal baseline data and imposed no criteria for making the diagnosis in the first place. The lack of diagnostic criteria could have led to bias from patient selection, but can be justified because there are no firm criteria on which Charcot diagnosis is made, it being essentially a clinical diagnosis confirmed by imaging (either X-ray or magnetic resonance imaging).
The principal limitation of the CDUK data is that they are simply observational, and it is not possible to use them as evidence of cause and effect with confidence. Differences may be the result of factors that have not been identified – even though the authors did their best to exclude those that might be predicted.
Other recent relevant publications
In order to demonstrate likely cause and effect with more certainty, it is necessary to undertake a randomised controlled trial, and one such trial has now been reported by Pakareinen and colleagues (2011) in Finland. Although only a pilot study of a small (n=39), but consecutive, series of acute Charcot cases, the authors compared zoledronic acid with placebo and reported that off-loading duration was significantly longer in those who received a bisphosphonate than in those who did not (27 weeks vs 20 weeks; P=0.02). Evidence from Pakareinen et al (2011) and CDUK taken together strongly suggest that the use of bisphosphonates is not beneficial in the acute Charcot foot. The same conclusion was reached in a recent systematic review published by Richard and colleagues (2012).
Conclusions
A number of conclusions can be drawn from the CDUK study when the findings are taken in the context of the related literature:
- There is currently no evidence to support the use of bisphosphonates in the management of the acute Charcot foot, and management currently rests on the use of effective off-loading.
- Only 35% of cases surveyed were treated according to current best practice (i.e. using a non-removable off-loading device). The implications of this need careful consideration.
- In the 6 months prior to presentation, there was a high prevalence of identified episodes of trauma that could have acted as the trigger to cycles of inflammation and bone breakdown in susceptible individuals.
- Although web-based audit has its limitations, it may yield valuable data on rare conditions in which it is difficult to undertake definitive trials in a single centre.
Acknowledgements
The CDUK study was planned and undertaken by the authors together with Michael Edmonds, Ed Jude, Geraint Jones and Gerry Rayman, with the help of Ryan Catlow, and was funded by Diabetes UK.
The authors are extremely grateful to the clinicians who contributed from each of the centres listed below.
CDUK contributors
Addenbrooke’s Hospital, Cambridge; Beaumont Hospital, Dublin; Burnley General Hospital, Burnley; Royal Blackburn Hospital, Blackburn; Bolton Diabetes Centre, Bolton; Bradford Royal Infirmary, Bradford; Brighton General Hospital, Brighton; Broomfield Hospital, Chelmsford; Bristol Royal Infirmary, Bristol; Buckinghamshire Podiatrist Service; Cannock Chase Hospital, Cannock; City Hospital, Nottingham; City Hospitals, Sunderland; Clifford Road Health Centre; Countess Of Chester Hospital; Cork University Hospital; Colchester Primary Care Centre; Darent Valley Hospital, Dartford; Derriford Hospital, Plymouth; Derby Royal Infirmary, Derby; East Surrey Hospital, Redhill; Epsom General Hospital, Epsom; George Eliot Hospital, Nuneaton; Glan Clwyd Hospital, Rhyl; Gloucester Royal Hospital, Gloucester; Glasgow Royal Infirmary, Glasgow; Grantham & District Hospital, Grantham; Hemel Hempstead Hospital, Hemel Hempstead; Hope Hospital, Salford; Hull Royal Infirmary, Hull; Ipswich Hospital, Ipswich; Kings College Hospital, London; King Edward VII Hospital, Windsor; King George’s Hospital Essex, Ilford; Lake Road Health Centre, Southsea; Leeds General Infirmary, Leeds; Leighton Hospital, Crewe; Lothian University Hospital, Edinburgh; Mile End Hospital, London; Middlesex Hospital, London; Milton Keynes Primary Care Trust; New Cross Hospital, London; Northern General Hospital, Sheffield; Northampton General Hospital, Northampton; Ninewells Hospital, Dundee; Northumbria Diabetes Service; Norfolk and Norwich University Hospital, Norwich; Peterborough City Clinic, Peterborough; Podiatry Service Nithbank, Dumfries and Galloway; Prince Phillip Hospital, Llanelli; Queen Margaret Hospital, Dunfermline; Royal Devon and Exeter Foundation Trust, Exeter; Royal Bournemouth Hospital, Bournemouth; Royal Glamorgan Hospital, Llantrisant; Royal United Hospital, Bath; Scunthorpe General Hospital, Scunthorpe; Darlington Memorial Hospital, Darlington; South Glasgow University Hospitals, Southmead Hospital, Bristol; South Tyneside District Hospital, South Shields; South West Kent PCT; St Richards Hospital, Chichester; St Thomas’ Hospital, London; Torbay Hospital, Torbay; Trafford Primary Care Trust, Manchester; University Hospitals of Leicester, Leicester; University Hospital Of Wales, Cardiff; University Of Ulster and Belfast City Hospital, Belfast; Victoria Centre, Romford; Whiston Hospital, Prescot; Woolmanhill Hospital, Aberdeen; Wycombe Hospital, High Wycombe; Wythenshawe Hospital, Manchester; York Hospital, York.