The nature of diabetic kidney disease
Diabetic kidney disease (DKD) is a term used to describe chronic kidney disease (CKD) in a person with diabetes in whom the CKD is attributable to diabetes-related pathology. It is typically a progressive condition associated with adverse renal and cardiovascular outcomes, potentially occurring in all forms of diabetes. DKD is characterised by:
- A gradually falling glomerular filtration rate (eGFR; a measure of kidney function), and/or
- A steadily rising albuminuria, usually defined by urinary albumin:creatinine ratio (uACR; a measure of renal damage) (de Boer et al, 2022).
- DKD is present if either eGFR is <60 mL/min/1.73 m2 or uACR is ≥3 mg/mmol, or both occur.
The fall in eGFR, indicates a reduction in effective filtration area of the glomeruli. A rise in uACR, represents leakage of albumin molecules through a damaged glomerular basement membrane.
Diabetic nephropathy refers specifically to pathology within the renal glomeruli as a consequence of chronic hyperglycaemia. Alongside diabetic retinopathy and diabetic neuropathy, it represents a microvascular complication of diabetes. A definitive diagnosis of diabetic nephropathy requires a renal biopsy, but these are not routinely carried out (KDIGO, 2022). People with diabetes are predisposed to acquire renal damage from causes other than diabetic nephropathy, including hypertensive renal disease and ischaemia arising from atherosclerotic disease affecting renal arteries. Thus, the pathology of DKD may be multifactorial.
People with diabetes may develop CKD that is not related to their diabetes. However, in the presence of retinopathy, CKD is very likely to be a consequence of diabetes, especially for people with type 1 diabetes (He et al, 2013; ADA, 2025). Box 1 shows some criteria for suggesting an alternative underlying cause of CKD, rather than diabetes. Diagnoses under consideration here would include vasculitic conditions, recurrent upper urinary tract infections, obstructive uropathy and myeloma. In these situations further blood tests, renal ultrasound and specialist referral may be required (Lewis, 2023).

Prevalence of DKD
In Europe, people with diabetes are 2–5 times more likely to develop CKD than people without diabetes (Bruck et al, 2016). Using the criteria of eGFR <60 mL/min/1.73 m2 or uACR ≥3 mg/mmol, or both, DKD was identified in 32% of people with type 1 diabetes and 46% of people with type 2 diabetes in the UK National Diabetes Audit (Hill et al, 2014). DKD typically takes around 10 years to develop in type 1 diabetes, but may be present at the point of diagnosis in type 2 diabetes (ADA, 2025).
Both in the UK and worldwide, DKD is the leading cause of end-stage kidney disease (ESKD), being responsible for around half of cases (Hongeveen, 2022). As the global epidemic of type 2 diabetes evolves, the incidence of DKD will inevitably increase.
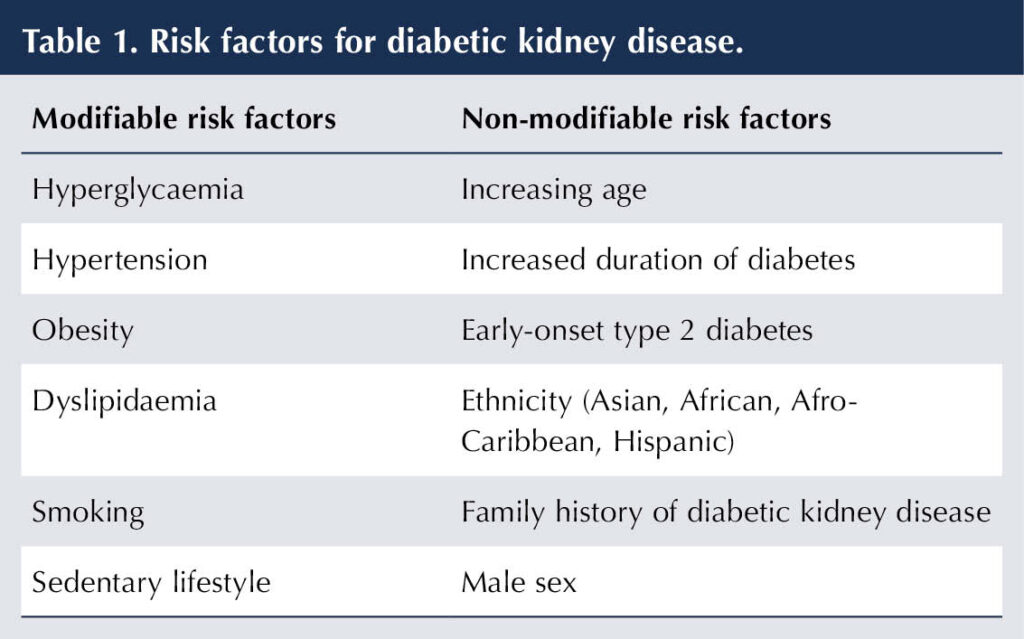
Risk factors for DKD
Hyperglycaemia and hypertension are key modifiable risk factors for the development of DKD in both type 1 and type 2 diabetes. Cardiovascular risk factors can also adversely affect renal function, with hypertension, dyslipidaemia and obesity being prominent in type 2 diabetes. Table 1 lists important modifiable and non-modifiable risk factors for DKD (Hongeveen, 2022).
Classification
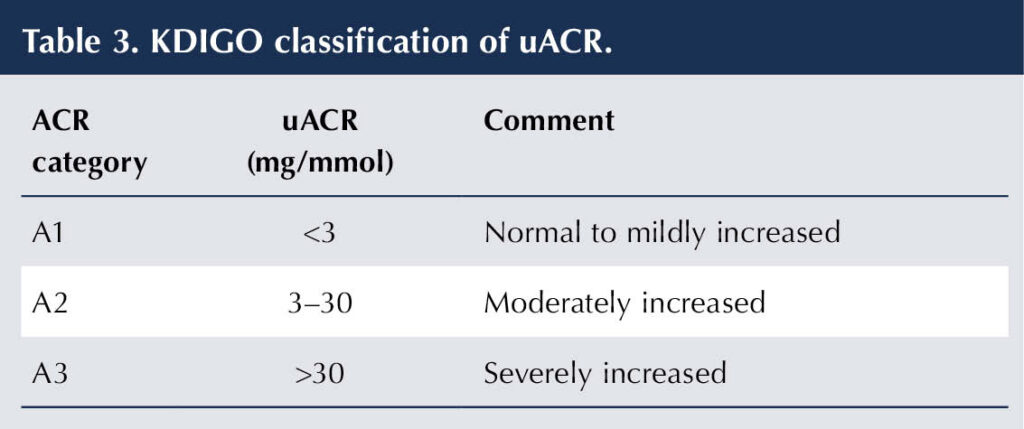
The KDIGO classification of CKD is based on eGFR and uACR (Table 2 and Table 3). For example, an individual with diabetes having an eGFR of 47 mL/min/1.73 m2 and an ACR of 12 mg/mmol would have DKD categorised as G3aA2 (KDIGO, 2022; de Boer et al, 2022).

Consequences of DKD
People with DKD are more likely to progress to ESKD than those without diabetes who have CKD. They are also at increased cardiovascular (CV) risk (myocardial infarction, ischaemic stroke or heart failure) compared to people with diabetes or CKD alone. Death from CV disease often precedes ESKD (NICE, 2021; KDIGO, 2022).
Both low eGFR and raised uACR are independently linked to an increased risk of CV and renal events, and all-cause mortality in people with type 2 diabetes. If both parameters are present, the risks are multiplied (Ninomiya et al, 2009; Toyama et al, 2013; NICE, 2021). The 10-year mortality risk for people with diabetes who have a uACR >3 mg/mmol and/or an eGFR <60 mL/min/1.73 m2 is four times greater than for those without diabetes or renal disease (Afkarian et al, 2013).
Individuals with DKD are at greater risk of treatment-induced hypoglycaemia (Moen et al, 2009) and acute kidney injury (AKI; James et al, 2010). Heavy proteinuria can generate nephrotic syndrome characterised by hypoalbuminaemia, peripheral oedema and hyperlipidaemia.
With more severe DKD (eGFR <30 mL/min/1.73 m2), impaired renal function has important consequences, including:
- Reduced production of renal erythropoietin, which can lead to anaemia.
- Reduced 1-alpha hydroxylation impedes production of 1,25 dihydroxyvitamin D3, the active metabolite responsible for calcium absorption. Hypocalcaemia can result.
- Secondary hyperparathyroidism in response to hypocalcaemia can lead to renal osteodystrophy (bone resorption, weakness and fracture).
- Reduced urinary excretion of phosphate can lead to hyperphosphataemia.
- Uraemia, causing fatigue, nausea, anorexia and pruritus (Bilous, 2016; NICE, 2021).
The personal consequences of having DKD can be profound. The requirement for dialysis or renal transplantation, and the cardiovascular and microvascular complications associated with DKD, inevitably impact adversely on quality of life. In low-income countries, in particular, the availability of kidney replacement therapy is limited. The economic burden of DKD is substantial and rising – by 2025 the cost of CKD per million individuals with diabetes in England has been forecast at approximately £11.4 bn (Nguyen et al, 2018).
Screening
Screening people with diabetes for DKD using eGFR and uACR is crucially important because, in its early stages, DKD is asymptomatic. An early diagnosis (see Table 3) offers the opportunity for intervention to delay progression DKD and reduce the risk of complications (NICE, 2021; 2024).
An eGFR <60 mL/min/1.73 m2 on at least two occasions separated by at least 90 days would, irrespective of uACR, be sufficient to make a diagnosis of DKD. If the initial eGFR measurement is unexpectedly low (or if this is the first eGFR measurement to be taken) and the question of AKI arises, then a repeat eGFR measurement should be taken within 2 weeks.
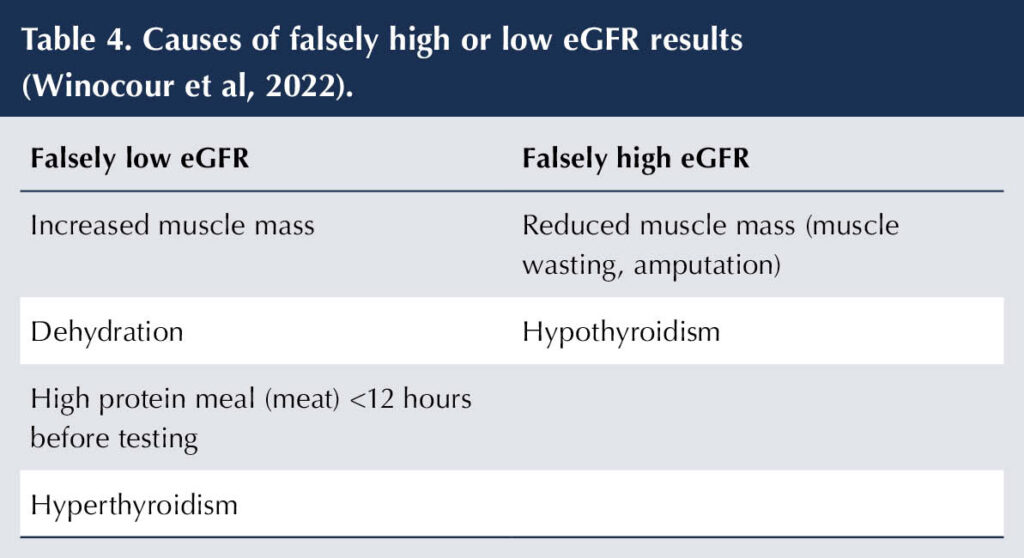
Situations in which eGFR can be misleading are shown in Table 4. Fluctuations in eGFR are common and repeat measurements should be taken if there are concerns. Changes in treatments commonly used in diabetes (e.g. ACE inhibitors/angiotensin receptor blockers, diuretics, SGLT2 inhibitors and NSAIDs) will impact on eGFR values. When the validity of a creatinine-based eGFR result is in doubt – and there are no other markers for DKD, such as albuminuria – then, depending on local arrangements, the diagnosis of DKD can be confirmed by measuring cystatin C. Cystatin C is a small protein that is produced at a steady rate and is freely filtered at the kidneys, making it less susceptible to variation than creatinine (Shiplak et al, 2013).
When a uACR sample is collected, a urinary dipstick test should be performed to exclude a false positive result for albuminuria secondary to a urinary tract infection (UTI) – look for leucocytes and nitrites. Further causes of falsely raised uACR include exercise, febrile illness, congestive cardiac failure and vaginal bleeding/discharge. A repeat elevated uACR (>3 mg/mmol) after 3/12 confirms DKD.
A positive dipstick test for haematuria should be repeated (whether or not there is proteinuria or a UTI) and, if persistent, should lead to referral for investigation of urological malignancies. If painless, macroscopic (visible) haematuria is reported, then urgent urology referral is indicated (NICE, 2023).
In the assessment of albuminuria:
- Ideally use an early morning urine (EMU) sample to assess uACR (less likely that exercise could induce a false positive).
- If the sample is not an EMU and uACR is in the range 3–70 mg/mmol, arrange for an EMU sample to be taken to confirm albuminuria as soon as is convenient. If uACR is >70 mg/mmol, a repeat sample is not necessary.
- A uACR of ≥3 mg/mmol is considered significant in people with diabetes (i.e. indicative of DKD).
- uACR is preferred to urinary protein/creatinine (uPCR) ratio because it is a more sensitive measure of low levels of proteinuria. If uACR is >70 mg/mmol, then uPCR is an adequate measure of proteinuria.
Monitoring
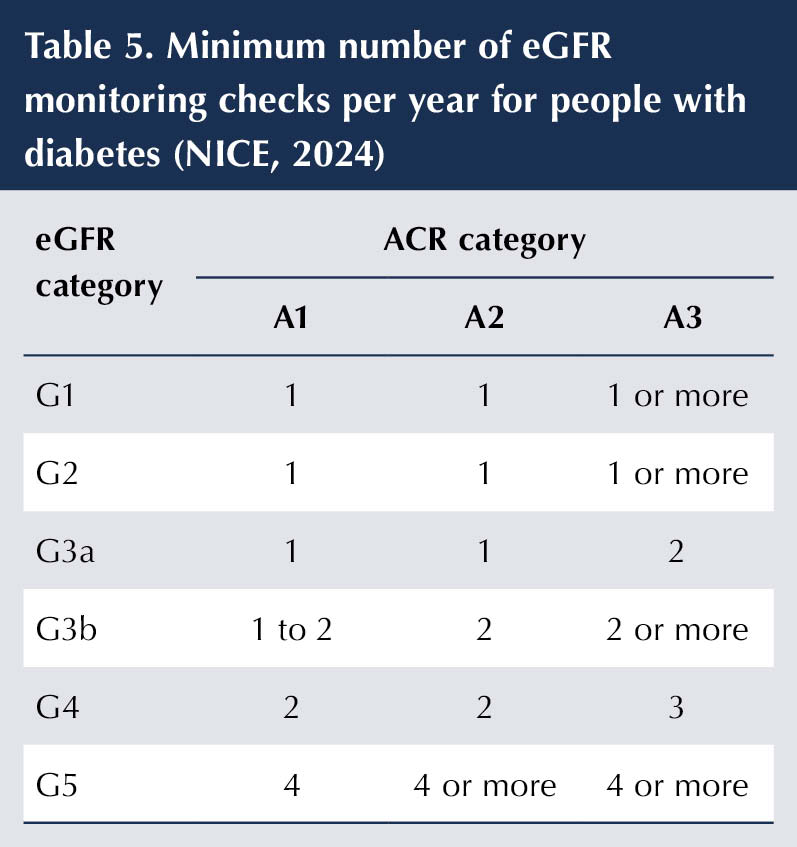
eGFR and uACR should be measured at least annually in people with diabetes to assess disease progression, inform on medical management and determine the need for referral (NICE, 2021; 2024). The frequency of eGFR monitoring in people with DKD should be determined by stage of kidney disease (see Table 5). There is less clear guidance on the how frequently uACR should be repeated, but the principle of testing more frequently in people at higher risk (i.e. lower eGFR and higher uACR) remains valid (Winocour et al, 2020; de Boer et al, 2022).
In advanced stages of DKD, there may be a need to conduct a full blood count for anaemia and test plasma levels of 25-hydroxyvitamin D levels, calcium, phosphate and parathyroid hormone, though by this stage the individual will usually be under specialist care.
When to refer
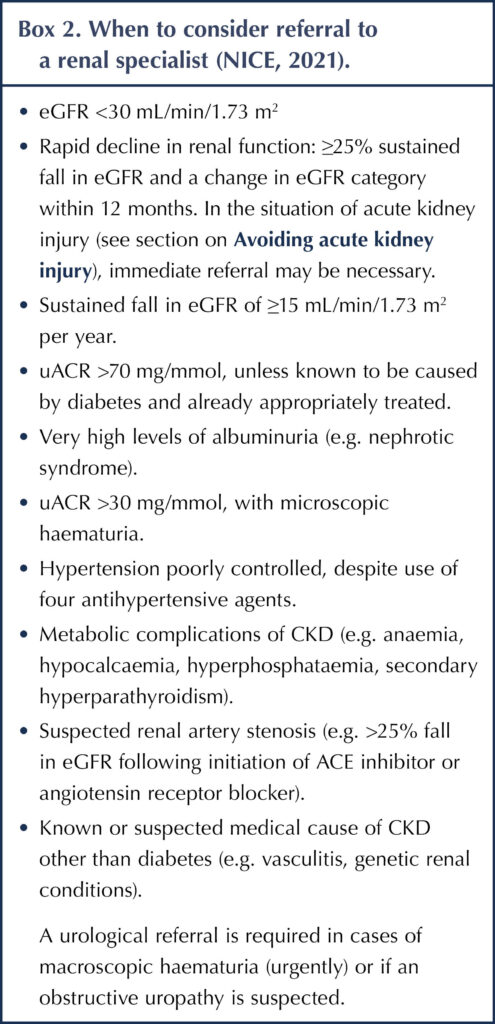
Significant progression of DKD, as judged by screening eGFR and uACR results or onset of metabolic problems, may require referral to a renal specialist (Box 2). Most cases of DKD can be managed in primary care.
Avoiding acute kidney injury
Acute kidney injury (AKI) is characterised by a sudden decline in eGFR, with onset of oliguria (low urine output). People with DKD are more vulnerable to AKI than those with CKD who do not have diabetes (NICE, 2019). It is important to be aware of the possibility of AKI, notably in situations of acute illness or volume depletion (e.g. diarrhoea and vomiting), and, where necessary, to check renal function.
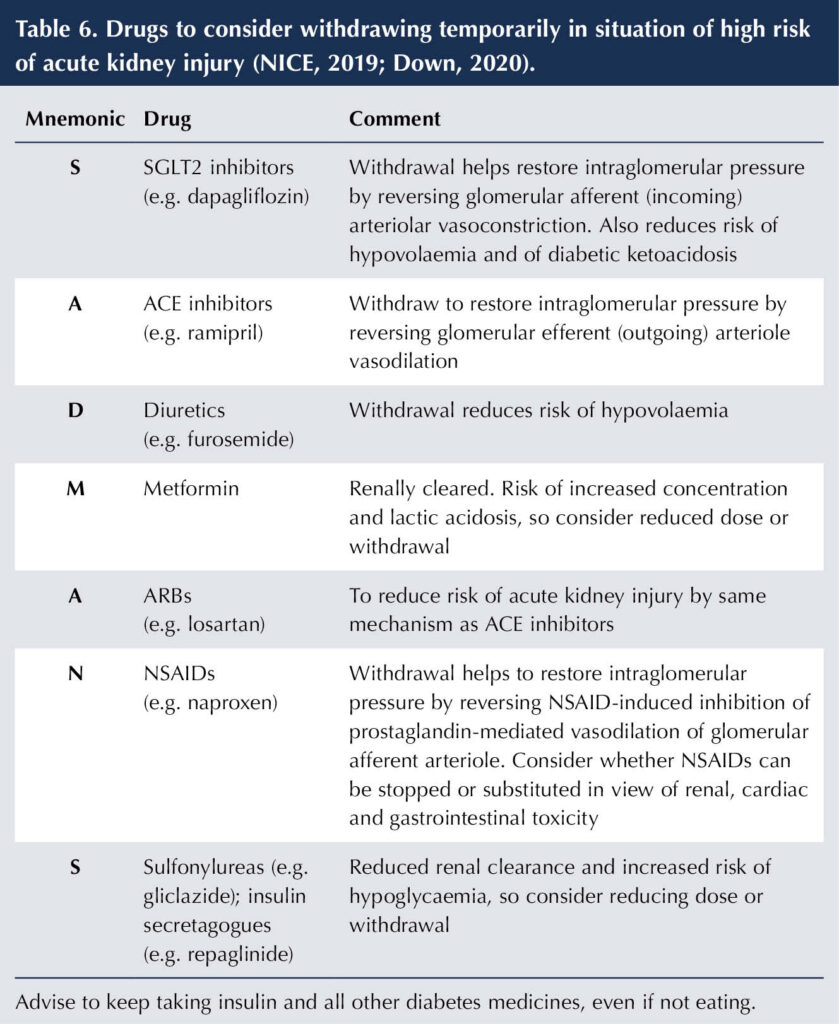
Under circumstances of acute illness and/or hypovolaemia, certain drugs commonly used in people with diabetes may need to be temporarily withdrawn to reduce the likelihood of AKI until the condition has resolved. The SADMANS pneumonic can be used as a reminder of the medications that can predispose to AKI (Table 6).
Conclusion
Diabetic kidney disease is a microvascular complication of diabetes affecting people with both type 1 and type 2 diabetes. It can have a profound impact on an individual’s quality of life. The risks of a cardiovascular event and the progression to ESKD are increased in people with DKD. The economic consequences of DKD are huge and the burden is increasing in line with the proliferation of type 2 diabetes.
DKD is the leading cause of ESKD, both in the UK and worldwide. It should be remembered, however, that there may be other causes of CKD in the individual with diabetes, and atypical features and/or rapidly deteriorating renal tests should prompt further investigation and referral to a renal specialist.
Screening using eGFR and urinary ACR in people with diabetes is crucial in identifying early DKD, providing the opportunity to reduce progression to more serious complications. Monitoring of eGFR and uACR guides interventions and the need for specialist referral.
People with DKD are at increased risk of AKI. During acute illness, be aware of the possibility of acute deterioration of renal function; it may be necessary to temporarily withdraw medications that predispose to AKI.





NHS type 2 diabetes prevention initiative reaches more people than ever.
5 Jun 2025