What are ACE inhibitors?
Angiotensin-converting enzyme inhibitors (ACE-Is) are widely used medications used in the treatment of hypertension, heart failure and chronic kidney disease, notably in the case of diabetic nephropathy. They have an important role in secondary prevention of cardiovascular disease post-myocardial infarction and for those who develop atherosclerotic cardiovascular disease (CVD).
ACE-Is are all given orally. In addition, enalapril has an intravenous formulation.
Mechanisms of action1
Effects on blood pressure
Inhibition of ACE lowers blood pressure in multiple ways:
● Blocking the conversion of angiotensin I to angiotensin II (a potent vasoconstrictor), thus facilitating vasodilation.
● Raising levels of bradykinin, a vasodilator.
● Lower levels of angiotensin II also result in decreased aldosterone levels, reducing sodium and, hence, water retention in the kidneys (natriuresis), leading to reduced blood volume.
Effects on heart failure
● The above vasodilatory effects reduce afterload, enabling increased cardiac output in heart failure.
● Myocyte hypertrophy is reduced, protecting against left ventricular dysfunction.
● Following myocardial infarction, the renin–angiotensin system is activated, and this can lead to left ventricular dilation and impaired function. ACE-Is counter these effects.
● ACE-Is also act centrally on the autonomic nervous system, inhibiting sympathetic activity, which protects against arrhythmias and the development of heart failure.
Renal effects
● Vasodilation of the glomerular efferent arterioles reduces intraglomerular pressure and preserves the glomerular basement membrane, thus ACE-Is have a renoprotective effect.
Licensed indications
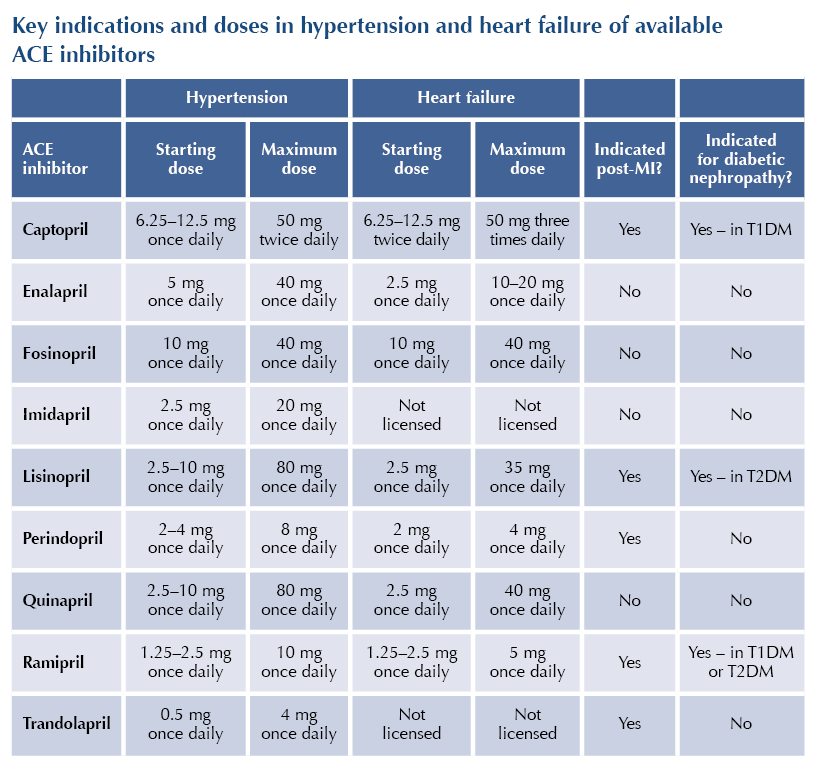
The licensed indications for ACE-Is vary between class members but include:
● Hypertension: alone or in conjunction with other antihypertensive agents.
● Heart failure or asymptomatic left ventricular systolic dysfunction.
● Post-myocardial infarction (MI): secondary prevention of cardiovascular disease and heart failure.
● Prevention of cardiovascular events in people with atherosclerotic cardiovascular disease or diabetes with at least one additional risk factor for cardiovascular disease.
● Diabetic nephropathy or non-diabetic nephropathy with proteinuria.
Positioning in guidelines
Hypertension
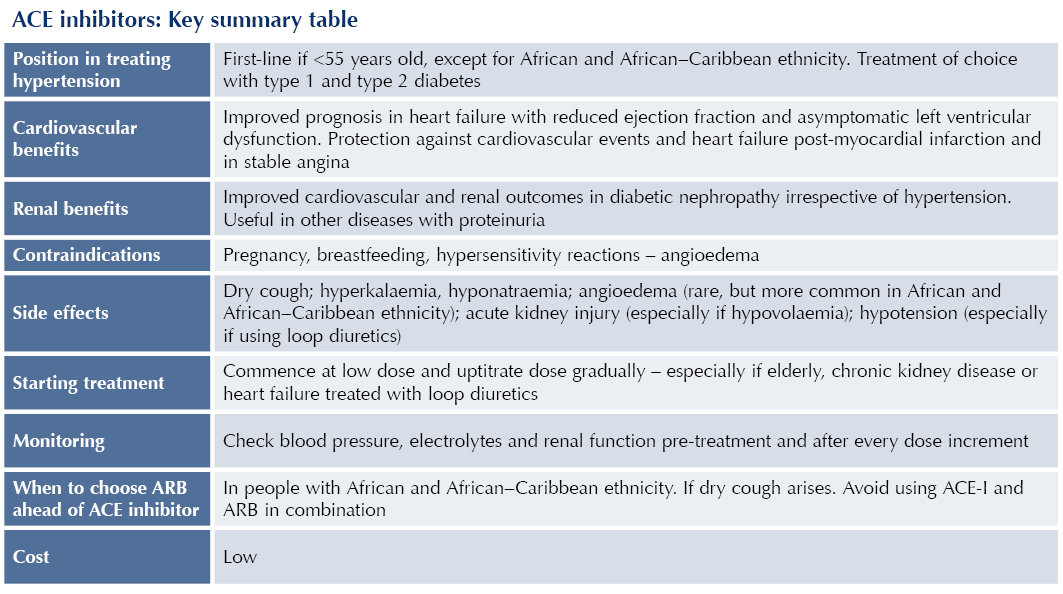
● NICE NG136 recommends ACE-Is (or angiotensin receptor blockers [ARBs]) as first-line antihypertensives in people <55 years old.2
- For those >55 years old and those with African or African–Caribbean ethnicity, calcium channel blockers (CCBs) should be offered as first-line antihypertensive agents, with thiazide-like diuretics an alternative if CCBs are not tolerated or contraindicated.
● ACE-Is (or ARBs) are the first-line treatment for hypertension for all adults with type 1 diabetes and type 2 diabetes because of their cardiorenal protective properties.2,3
● ADA guidelines recommend ACE-Is (or ARBs) as first-line antihypertensives for people with diabetes or established coronary artery disease.4
● ACE-Is are a second-line option for a person whose hypertension is not controlled with a CCB (or thiazide-like diuretic) and can also be added to a combination of a CCB and thiazide as part of triple therapy.2
● For people with African or African–Caribbean ethnicity, an ARB should be chosen ahead of an ACE-I.2
Heart failure (NICE NG106)5
● ACE-Is (or ARBs) should be offered as first-line treatment to individuals with heart failure with reduced ejection fraction (alongside a beta-blocker).
Chronic kidney disease (NICE NG203)6
● Whether or not hypertension is present, ACE-Is have an important role in treating chronic kidney disease (CKD), notably when albuminuria is present, to slow the progression of renal disease and reduce cardiovascular risk.
● For people with type 1 or type 2 diabetes, offer an ACE-I (or ARB) titrated to the highest tolerated dose if urinary albumin/creatinine ratio (uACR) is 3 mg/mmol or more.
● For people without diabetes, offer an ACE-I (or ARB) titrated to the highest tolerated dose if uACR is ≥70 mg/mmol. If hypertension is also present, offer if uACR is >30 mg/mmol.
● ADA guidelines recommend an ACE-I (or ARB) for people with diabetes who have albuminuria, to prevent the progression of renal disease.4
Acute coronary syndromes and stable angina (NICE NG185)7
● Offer people who have suffered a myocardial infarction an ACE-I (or ARB) as soon as they are haemodynamically stable for secondary prevention of cardiovascular disease (alongside dual antiplatelet therapy, beta-blockers and statins), and continue indefinitely.
● For individuals who have suffered a previous myocardial infarction, start an ACE-I (or ARB), titrate up to the maximum tolerated dose and continue indefinitely.
● ACE-Is (or ARBs) are indicated for the treatment of stable angina (NICE CG126).8
Principal effects
● ACE-Is lower BP by 11/6 mmHg within 12 hours following administration, with around 90% of maximal BP lowering occurring at half-maximal dose. No significant difference observed between class members.9
● In individuals with diabetes and hypertension with no prior history of coronary heart disease, ACE-Is reduce risk of myocardial infarction and non-fatal stroke, and improve left ventricular function.10
● In people with diabetic nephropathy, ACE-Is slow progression of proteinuria and stabilise renal function, with benefits over and above BP control alone.11
- Renoprotective properties extend to normotensive people with diabetes and proteinuria.12
- ACE-Is attenuate the decline in eGFR and proteinuria in non-diabetic nephropathy.1 However, in the absence of albuminuria, advantages over other antihypertensive agents are less clear.13
● ACE-Is improve symptoms and reduce morbidity and overall mortality in heart failure with reduced ejection fraction – reducing cardiovascular events and attenuating heart failure progression.14,15
- Asymptomatic individuals with left ventricular dysfunction also benefit from ACE-Is.
● ACE-Is reduce mortality and slow progression to heart failure following myocardial infarction.16,17
● Equivalent efficacy to ARBs in providing cardiovascular benefits and renoprotection both in people with and without diabetes.18–20
Contraindications
● Pregnancy and where pregnancy is planned: teratogenic effects including reduced fetal renal function and oligohydramnios.
● Breastfeeding: limited information.
● Hypersensitivity reactions: including previous angioedema with ACE-Is. Avoid ACE-Is if family history of hereditary angioedema or personal history of angioedema.
● Bilateral renal artery stenosis: risk of acute kidney injury (AKI).
Cautions
● Aortic or mitral valve stenosis: risk of severe hypotension.
● Hypertrophic cardiomyopathy.
● Hypovolaemia: ACE-Is can reduce renal function and predispose to AKI and hyperkalaemia under these circumstances.
● High-dose diuretic therapy (>80 mg furosemide or equivalent).
● Low systolic blood pressure (<100 mmHg): risk of hypotension.
Drug interactions
● Avoid using ACE-Is and ARBs in combination – outcomes are not improved and there is increased risk of hyperkalaemia and AKI.21,22
● The combination of ACE-Is and the direct renin inhibitor aliskiren is not recommended and is contraindicated in people with diabetes or an eGFR <60 mL/min/1.73 m2.
Adverse effects
● Dry cough (10–20%): due to accumulation of bradykinin, as a result of reduced metabolism by ACE. Cough resolves within 1 month of stopping ACE-I. Consider switch to ARB, which carries lower risk of cough.
● Hyperkalaemia: secondary to reduced levels of aldosterone.
- Higher risk if other potassium-retaining medications are being used, such as potassium-retaining diuretics (e.g. amiloride) and mineralocorticoid receptor antagonists (e.g. spironolactone).23
- More likely if initial eGFR is <45 mL/min/1.73 m2, or if initial potassium level is >4.5 mmol/L.
● Hyponatraemia: secondary to reduced levels of aldosterone.
● Angioedema: rare, but potentially life-threatening swelling of face, lips and upper airway.
- Increased risk in people of African and African–Caribbean origin, so consider starting an ARB in preference to an ACE-I in this ethnic group.
- If angioedema experienced with ACE-I then avoid ARB.2
● Acute kidney injury: higher risk if pre-existing CKD or renal artery stenosis; can be precipitated by hypovolaemia.
● Hypotension: higher risk of first-dose hypotension in individuals with heart failure and low systolic blood pressure taking high dose of loop diuretics (e.g. furosemide).
- Start with very low dose of ACE-I under these circumstances.
Prescribing tips
Initiation
● Have available baseline electrolytes and renal function before starting an ACE-I.
● Start ACE-Is at low dose and titrate the dose up at short intervals (e.g. every 2–4 weeks), until the maximum tolerated or target dose is achieved.
● Use lower starting doses and titrate doses up more slowly in the elderly or those with CKD (notably if eGFR is <45 mL/min/1.73 m2).
● Avoid starting ACE-Is if baseline potassium is >5 mmol/L. If necessary, consider altering any potassium-retaining medication (e.g. potassium-sparing diuretics) to allow use of ACE-I.
Monitoring
● Monitor blood pressure before and after each dose increment of ACE-I.
● Measure serum electrolytes and renal function 1–2 weeks after starting an ACE-I and after each dose increment.
eGFR
● A cumulative fall in eGFR of <25% or a rise in serum creatinine of <30% from baseline are acceptable. If these are exceeded, look for other possible causes, including hypovolaemia or concurrent medication (e.g. NSAIDs) before stopping or reducing dose of the ACE-I.
● If there is acute kidney injury (very low eGFR), stop ACE-I and refer immediately to hospital.
Potassium
● If serum potassium is 5 mmol/L or more, look for other contributors to hyperkalaemia and change treatment as appropriate.
● A potassium level of up to 5.5 mmol/L is acceptable. If 5.5–6.0 mmol/L despite taking measures to reduce levels, reduce dose of ACE-I to achieve potassium levels that are acceptable.
● If potassium rises to >6 mmol/L and other potassium-retaining medications have been discontinued where possible, stop ACE-I and recheck K level; if ≥6.5 mmol/L, or ECG changes, refer immediately to hospital.
Sick day rules
● Temporarily withdraw ACE-I if hypovolaemia (e.g. diarrhoea and vomiting) to reduce risk of AKI and hyperkalaemia – increased risk if taking loop diuretics.
Key summary table






Satish Durgam reviews who will be eligible to receive tirzpepatide for weight management and when.
24 Apr 2025