A relationship between long-term metformin use and vitamin B12 deficiency has been long discussed, and many readers will have come across this in clinical practice; however, until now, we have been without any official guidance on management. In June 2022, the MHRA published advice, following a review in Europe, which concluded that this side effect occurs more frequently than was previously thought (MHRA, 2022). This has led to an update to the Product Information for all metformin-containing medications.
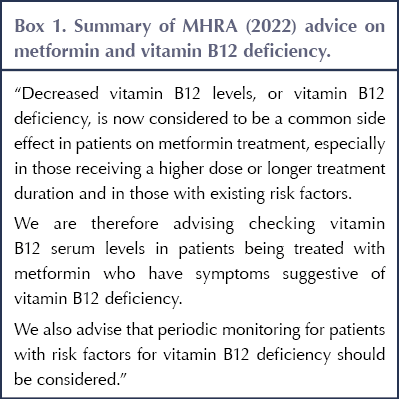
The MHRA states that low vitamin B12 is now considered to be a common side effect, especially when taking high-dose or long-term metformin, affecting up to one in 10 people. It advises checking levels in patients with symptoms of B12 deficiency, as well as monitoring those at risk of B12 deficiency (see Box 1).
What is the evidence?
As far back as the 1970s, there were case reports in the literature of B12 deficiency associated with metformin use, with several observational studies reporting the same, but very little in the way of high-quality, well-powered, prospective studies. The Diabetes Prevention Program was a large, long-term study enrolling people with pre-diabetes, with one arm using metformin. In a post hoc analysis after 13 years of the study, a 13% increased risk of vitamin B12 deficiency per year of metformin use was reported (Aroda et al, 2016).
In 2010, a randomised, placebo-controlled trial was published in the BMJ. This enrolled 390 people with type 2 diabetes, all on insulin, and randomised them to metformin 850 mg three times daily or placebo, with a follow-up of just over 4 years (de Jager et al, 2010). This trial demonstrated a mean decrease in B12 levels of 19%, with a number needed to harm of 13.8 over 4.3 years; that is, if 14 patients were treated for 4 years with metformin 850 mg t.d.s., one would develop vitamin B12 deficiency. Importantly, this study also demonstrated that the B12 reduction associated with metformin use is not transitory but persists and progresses over time and, in some patients, to levels at which replacement is recommended.
A retrospective study looking at concomitant use of proton pump inhibitors (PPIs) alongside metformin in 614 people with type 2 diabetes in the US found a significant reduction in B12 levels of 34% in patients taking both medications, compared to controls taking either drug alone (Long et al, 2012).
Why does it happen?
Vitamin B12 deficiency associated with metformin use is thought to be multifactorial. Theories include alteration of bile acid metabolism, small intestinal bacterial overgrowth, effects on intestinal motility and interference by metformin on vitamin B12-intrinsic factor absorption in the terminal ileum. The use of PPIs is also thought to contribute to vitamin B12 deficiency (Aroda et al, 2016).
What should we do in clinical practice?
Check vitamin B12 levels in people treated with metformin who have symptoms suggestive of vitamin B12 deficiency
This will also include patients found to have megaloblastic anaemia (raised mean corpuscular volume [MVC]), who may be asymptomatic (MHRA, 2022). In my own clinical practice, I already recommend a full blood count as part of annual review, to ensure that the person is not anaemic and, thus, that the HbA1c is reliable, but this also proves useful to assess MCV in this context. If a person has a raised MCV, I will go on to check vitamin B12 levels.
Symptoms of B12 deficiency include the following (MHRA, 2022):
- Neuropathy.
- Mental disturbance (depression, irritability, cognitive impairment).
- Glossitis (swollen and inflamed tongue).
- Mouth ulcers.
- Visual and motor disturbances.
Investigation of these symptoms in a person taking metformin would definitely prompt checking their B12 level. The MHRA advice reminds us that in a person with anaemia or neuropathy caused by vitamin B12 deficiency, it is important that treatment starts as soon as possible, in order to avoid the development of permanent symptoms.
Consider periodic monitoring of B12 in patients on metformin with risk factors for B12 deficiency
Risk factors are quite wide-ranging and include the following (MHRA, 2022):
- Baseline vitamin B12 levels at the lower end of the normal range.
- Factors and conditions associated with reduced B12 absorption, including old age; gastrointestinal disorders such as total or partial gastrectomy, Crohn’s disease and other bowel inflammatory disorders; and autoimmune conditions.
- Diets with reduced sources of vitamin B12, such as strict vegan and some vegetarian diets.
- Concomitant medications known to impair vitamin B12 absorption, including PPIs and colchicine.
- Genetic predisposition to B12 deficiency, such as intrinsic factor receptor deficiency (Imerslund–Gräsbeck syndrome) and transcobalamin II deficiency.
In these groups of patients with risk factors who are taking metformin, especially over the long term, the new MHRA guidance suggests we should consider checking vitamin B12 levels as part of annual review bloods – this is a significant change to current typical practice.
What if vitamin B12 is found to be low in a person taking metformin?
The advice is to investigate and treat the vitamin B12 deficiency in the normal way, following clinical guidelines, and to continue the metformin. We know that metformin is a very useful medication in type 2 diabetes, combating insulin resistance, and is recommended first-line in almost all national and international guidelines. Replacing vitamin B12 is relatively straightforward and risk-free – although it can be resource-heavy for primary care when regular intramuscular therapy is required.
Treating B12 deficiency in a person taking metformin
There is no clear guidance on the best way to treat vitamin B12 deficiency in a person taking metformin. The MHRA advises following current clinical guidelines, and it signposts to the NICE Clinical Knowledge Summary (CKS) on this topic (NICE, 2022). Many local guidelines on B12 replacement therapy are also available.
We can replace B12 either orally or via intramuscular injections. The mechanisms behind B12 deficiency in metformin users are multifactorial but are probably related to intestinal absorption, so it seems sensible to assume that oral B12 replacement may be less effective, although there is little evidence in this area. However, regular intramuscular injections are a significant strain on resources (both time and financial) and are inconvenient for patients, so a trial of oral replacement therapy may be suitable in some cases. Unfortunately, as there is no clear guidance, consideration on a patient-by-patient basis is required. My own clinical practice is usually a trial of oral replacement initially, checking levels as per the monitoring advice below, and switching to intramuscular replacement where indicated.
The general CKS guidance for vitamin B12 replacement is summarised in Box 2. Most of our patients on metformin with B12 deficiency will fall into the “not diet-related” category and, therefore, many will eventually require intramuscular replacement.

Monitoring
For patients with symptomatic B12 deficiency (e.g. neuropathy), effective replacement therapy should lead to a normal full blood count and B12 level after 8 weeks of treatment, while improvement in neurological symptoms begins very quickly – within one week of initiation of therapy – but can take up to 3 months to resolve (Carmel, 2008).
The NICE (2022) CKS suggests that measuring vitamin B12 levels is unhelpful because they will rise regardless of effectiveness; however, it does suggest checking 1–2 months after treatment if there is no clinical response.
If oral B12 replacement has been trialled in a person with metformin-associated B12 deficiency, I would suggest that a repeat measurement of B12 levels after 8–12 weeks is a good time to reassess whether absorption is adequate or whether a switch to intramuscular replacement is required. If a person has been commenced on intramuscular replacement, there is no need to recheck levels.
After commencement of replacement therapy, once adequate absorption has been confirmed if required, no further monitoring needs to be undertaken and the individual will continue replacement therapy for the duration of their metformin treatment (Stabler, 2013).
Conclusions
I welcome this clear advice from the MHRA, clarifying the frequency of vitamin B12 deficiency in patients taking metformin, especially those on higher doses and longer-term therapy.
It is a useful reminder to consider vitamin B12 deficiency in people taking metformin who have megaloblastic anaemia, neuropathy or other symptoms suggestive of deficiency. The biggest change to current clinical practice is likely to be the advice to consider periodic monitoring of B12 levels in patients taking metformin who are at risk of deficiency, which includes a wide range of our patients living with type 2 diabetes.
The guidance around the management of B12 deficiency in people treated with metformin is a little unclear, owing to a lack of clinical evidence in this area. It is likely that many patients will require intramuscular B12 replacement for the duration of their metformin treatment, although a trial of oral replacement initially may be worthwhile.







Case study outlining the complexities of managing glycaemia in a pregnant woman with type 1 diabetes.
30 May 2025